Rates and Microbial Players of Iron-Driven Anaerobic Oxidation of Methane in Methanic Marine Sediments
- PMID: 32010098
- PMCID: PMC6979488
- DOI: 10.3389/fmicb.2019.03041
Rates and Microbial Players of Iron-Driven Anaerobic Oxidation of Methane in Methanic Marine Sediments
Abstract
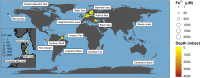
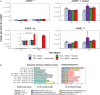
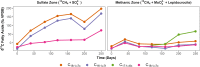
The flux of methane, a potent greenhouse gas, from the seabed is largely controlled by anaerobic oxidation of methane (AOM) coupled to sulfate reduction (S-AOM) in the sulfate methane transition (SMT). S-AOM is estimated to oxidize 90% of the methane produced in marine sediments and is mediated by a consortium of anaerobic methanotrophic archaea (ANME) and sulfate reducing bacteria. An additional methane sink, i.e., iron oxide coupled AOM (Fe-AOM), has been suggested to be active in the methanic zone of marine sediments. Geochemical signatures below the SMT such as high dissolved iron, low to undetectable sulfate and high methane concentrations, together with the presence of iron oxides are taken as prerequisites for this process. So far, Fe-AOM has neither been proven in marine sediments nor have the governing key microorganisms been identified. Here, using a multidisciplinary approach, we show that Fe-AOM occurs in iron oxide-rich methanic sediments of the Helgoland Mud Area (North Sea). When sulfate reduction was inhibited, different iron oxides facilitated AOM in long-term sediment slurry incubations but manganese oxide did not. Especially magnetite triggered substantial Fe-AOM activity and caused an enrichment of ANME-2a archaea. Methane oxidation rates of 0.095 ± 0.03 nmol cm-3 d-1 attributable to Fe-AOM were obtained in short-term radiotracer experiments. The decoupling of AOM from sulfate reduction in the methanic zone further corroborated that AOM was iron oxide-driven below the SMT. Thus, our findings prove that Fe-AOM occurs in methanic marine sediments containing mineral-bound ferric iron and is a previously overlooked but likely important component in the global methane budget. This process has the potential to sustain microbial life in the deep biosphere.
Keywords: ANME-2a; anaerobic methanotrophs; anaerobic oxidation of methane; iron oxides; marine sediment; microbial community analysis; radiotracer; stable isotope probing.
Copyright © 2020 Aromokeye, Kulkarni, Elvert, Wegener, Henkel, Coffinet, Eickhorst, Oni, Richter-Heitmann, Schnakenberg, Taubner, Wunder, Yin, Zhu, Hinrichs, Kasten and Friedrich.
Figures



References
-
- Aller R. C., Mackin J. E., Cox R. T. (1986). Diagenesis of Fe and S in Amazon inner shelf muds: apparent dominance of Fe reduction and implications for the genesis of ironstones. Cont. Shelf Res. 6 263–289. 10.1016/0278-4343(86)90064-6 - DOI
-
- Aromokeye D. A. (2018). Iron Oxide Driven Methanogenesis and Methanotrophy in Methanic Sediments of Helgoland Mud Area, North Sea. Doctoral dissertation, University of Bremen, Bremen.
-
- Aromokeye D. A., Richter-Heitmann T., Oni O. E., Kulkarni A., Yin X., Kasten S., et al. (2018). Temperature controls crystalline iron oxide utilization by microbial communities in methanic ferruginous marine sediment incubations. Front. Microbiol. 9:2574. 10.3389/fmicb.2018.02574 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

