Differences in Gut Microbial and Serum Biochemical Indices Between Sows With Different Productive Capacities During Perinatal Period
- PMID: 32010103
- PMCID: PMC6978668
- DOI: 10.3389/fmicb.2019.03047
Differences in Gut Microbial and Serum Biochemical Indices Between Sows With Different Productive Capacities During Perinatal Period
Abstract
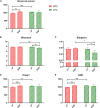
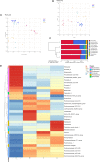
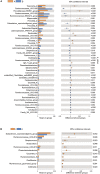
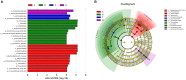
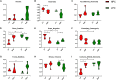
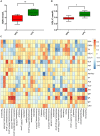
Maternal gut microflora changes dramatically during perinatal period and plays a vital role in animal health and reproductive performance. However, little is known about the microbial differences between sows with different productive capacities during perinatal period. Hence, this study explored fecal microbial diversity, composition, metabolic functions, and phenotypes differences between high productive capacity (HPC, litter size ≥ 15) and low productive capacity (LPC, litter size ≤ 7) sows during late pregnancy (LP, the third day before due date) and early stage after parturition (EAP, the third day after parturition) as well as serum biochemical indices differences after parturition. Results showed that HPC sows had lower microbial richness at LP stage and higher microbial diversity at EAP stage than LPC sows. Several genera belonging to the Prevotellaceae family exhibited higher abundance, while some genera belonging to the Ruminococcaceae family exhibited lower abundance in HPC sows compared to LPC sows at LP stage. Moreover, the relative abundance of Eubacterium_coprostanoligenes_group and Ruminococcaceae_UCG-014 in HPC sows was significantly higher than that in LPC sows at EAP stage. The predicted metabolic functions related to Lipopolysaccharide biosynthesis were significantly higher in HPC sows at LP stage. Further, HPC sows had significantly higher blood urea nitrogen (BUN) and high-density lipoprotein cholesterol (HDL-C) levels after parturition, and there were strong correlations between BUN level and the relative abundance of genera belonging to the Ruminococcaceae families. These results indicated that the HPC sows may experience greater inflammation than LPC sows at LP stage. Inflammation environment might impact health but promote parturition. The microbial differences at EAP stage might be beneficial to hemostasis and anti-inflammation, which might contribute to postpartum recovery in HPC sow.
Keywords: gut microbiota; perinatal period; productive capacity; serum immunity; sows.
Copyright © 2020 Shao, Zhou, Xiong, Zou, Kong, Tan and Yin.
Figures

References
-
- Alexopoulos C., Georgoulakis I., Tzivara A., Kritas S., Siochu A., Kyriakis S. (2004). Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J. Anim. Physiol. Anim. Nutr. 88 381–392. 10.1111/j.1439-0396.2004.00492.x - DOI - PubMed
-
- Bäckström L. (1973). Environment and animal health in piglet production. a field study of incidences and correlations. Acta Vet. Scand. Suppl. 41 1–240. - PubMed
LinkOut - more resources
Full Text Sources

