egc Superantigens Impair Monocytes/Macrophages Inducing Cell Death and Inefficient Activation
- PMID: 32010128
- PMCID: PMC6974467
- DOI: 10.3389/fimmu.2019.03008
egc Superantigens Impair Monocytes/Macrophages Inducing Cell Death and Inefficient Activation
Abstract
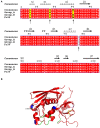
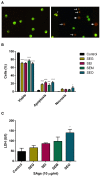
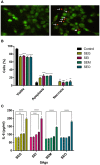
Bacterial superantigens (SAgs) are enterotoxins that bind to MHC-II and TCR molecules, activating as much as 20% of the T cell population and promoting a cytokine storm which enhances susceptibility to endotoxic shock, causing immunosuppression, and hindering the immune response against bacterial infection. Since monocytes/macrophages are one of the first cells SAgs find in infected host and considering the effect these cells have on directing the immune response, here, we investigated the effect of four non-classical SAgs of the staphylococcal egc operon, namely, SEG, SEI, SEO, and SEM on monocytic-macrophagic cells, in the absence of T cells. We also analyzed the molecular targets on APCs which could mediate SAg effects. We found that egc SAgs depleted the pool of innate immune effector cells and induced an inefficient activation of monocytic-macrophagic cells, driving the immune response to an impaired proinflammatory profile, which could be mediated directly or indirectly by interactions with MHC class II. In addition, performing surface plasmon resonance assays, we demonstrated that non-classical SAgs bind the gp130 molecule, which is also present in the monocytic cell surface, among other cells.
Keywords: MHC-II; THP-1; gp130; innate immune response; monocytes; superantigens.
Copyright © 2020 Noli Truant, De Marzi, Sarratea, Antonoglou, Meo, Iannantuono López, Fernández Lynch, Todone, Malchiodi and Fernández.
Figures


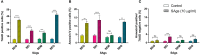



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

