Toxoplasma gondii Recruits Factor H and C4b-Binding Protein to Mediate Resistance to Serum Killing and Promote Parasite Persistence in vivo
- PMID: 32010145
- PMCID: PMC6979546
- DOI: 10.3389/fimmu.2019.03105
Toxoplasma gondii Recruits Factor H and C4b-Binding Protein to Mediate Resistance to Serum Killing and Promote Parasite Persistence in vivo
Abstract
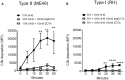
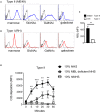
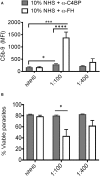
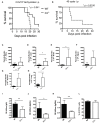
Regulating complement is an important step in the establishment of infection by microbial pathogens. Toxoplasma gondii actively resists complement-mediated killing in non-immune human serum (NHS) by inactivating C3b, however the precise molecular basis is unknown. Here, a flow cytometry-based C3b binding assay demonstrated that Type II strains had significantly higher levels of surface-bound C3b than Type I strains. However, both strains efficiently inactivated C3b and were equally resistant to serum killing, suggesting that resistance is not strain-dependent. Toxoplasma activated both the lectin (LP) and alternative (AP) pathways, and the deposition of C3b was both strain and lectin-dependent. A flow cytometry-based lectin binding assay identified strain-specific differences in the level and heterogeneity of surface glycans detected. Specifically, increased lectin-binding by Type II strains correlated with higher levels of the LP recognition receptor mannose binding lectin (MBL). Western blot analyses demonstrated that Toxoplasma recruits both classical pathway (CP) and LP regulator C4b-binding proteins (C4BP) and AP regulator Factor H (FH) to the parasite surface to inactivate bound C3b-iC3b and C3dg and limit formation of the C5b-9 attack complex. Blocking FH and C4BP contributed to increased C5b-9 formation in vitro. However, parasite susceptibility in vitro was only impacted when FH was blocked, indicating that down regulation of the alternative pathway by FH may be more critical for parasite resistance. Infection of C3 deficient mice led to uncontrolled parasite growth, acute mortality, and reduced antibody production, indicating that both the presence of C3, and the ability of the parasite to inactivate C3, was protective. Taken together, our results establish that Toxoplasma regulation of the complement system renders mice resistant to acute infection by limiting parasite proliferation in vivo, but susceptible to chronic infection, with all mice developing transmissible cysts to maintain its life cycle.
Keywords: Toxoplasma gondii; alternative pathway regulation; complement system resistance; immune evasion; protozoan parasites.
Copyright © 2020 Sikorski, Commodaro and Grigg.
Figures



References
-
- Morgan BP. The human complement system in health and disease. Ann Rheum Dis. (1998) 57:581–1. 10.1136/ard.57.10.581 - DOI
-
- Evans-Osses I, de Messias-Reason I, Ramirez MI. The Emerging Role of Complement Lectin Pathway in Trypanosomatids: Molecular Bases in Activation, Genetic Deficiencies, Susceptibility to Infection, and Complement System-Based Therapeutics. In: The Scientific World Journal. (2013). Available online at: https://www.hindawi.com/journals/tswj/2013/675898/ (accessed xMay 21, 2019). 10.1155/2013/675898 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

