Endogenous T Cell Receptor Rearrangement Represses Aggressive Central Nervous System Autoimmunity in a TcR-Transgenic Model on the Non-Obese Diabetic Background
- PMID: 32010149
- PMCID: PMC6974510
- DOI: 10.3389/fimmu.2019.03115
Endogenous T Cell Receptor Rearrangement Represses Aggressive Central Nervous System Autoimmunity in a TcR-Transgenic Model on the Non-Obese Diabetic Background
Abstract
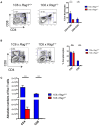
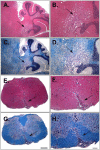
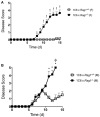
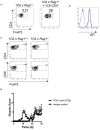
The T cell response to central nervous system (CNS) antigen in experimental autoimmune encephalomyelitis (EAE) permits one to model the immune aspects of multiple sclerosis. 1C6 transgenic mice on the non-obese diabetic (NOD) background possess a class II-restricted T cell receptor (TcR; Vα5-Vβ7) specific for the encephalitogenic peptide myelin oligodendrocyte glycoprotein (MOG)[35-55]. It remains to be determined what role is played by allelic inclusion in shaping the TcR repertoire of these mice. Here, we show that 1C6 T cells display substantial promiscuity in their expression of non-transgenically derived Vα chains. Further, enforced expression of the transgenic TcR in 1C6 × Rag1-/- mice profoundly disrupted thymic negative selection and led to a sharp decrease in the number of mature peripheral T cells. 1C6 × Rag1-/- mice developed spontaneous EAE at a significant frequency and rapidly developed fatal EAE upon immunization with myelin oligodendrocyte glycoprotein (MOG)[35-55]. Passive transfer of 1C6 × Rag1+/+ CD4+ T cells, but not CD8+ T cells or B cells, partially rescued 1C6 × Rag1-/- mice from severe EAE. FoxP3+ CD4+ Treg cells were present in the CNS of immunized 1C6 mice, as well as immunized 1C6 × Rag1-/- that had been supplemented with 1C6 CD4+ T cells. However, they were not observed in 1C6 × Rag1-/- that did not receive Rag1-sufficient 1C6 CD4+. Further, in vivo blockade of Treg accelerated the onset of symptoms in 1C6 mice immunized with MOG[35-55], indicating the pertinence of Treg-mediated control of autoimmune inflammation in this model. Thus, TcR allelic inclusion is crucial to the generation of FoxP3+ CD4+ T cells necessary for the suppression of severe CNS autoimmunity.
Keywords: 1C6; EAE (experimental autoimmune encephalomyelitis); FoxP3; RAG; TCR transgenic mice; Treg—regulatory T cell; allelic exclusion; non-obese diabetic (NOD).
Copyright © 2020 Yeola, Ignatius Arokia Doss, Baillargeon, Akbar, Mailhot, Balood, Talbot, Anderson, Lacroix and Rangachari.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

