Expression Pattern and Functional Characterization of PISTILLATA Ortholog Associated With the Formation of Petaloid Sepals in Double-Flower Eriobotrya japonica (Rosaceae)
- PMID: 32010167
- PMCID: PMC6978688
- DOI: 10.3389/fpls.2019.01685
Expression Pattern and Functional Characterization of PISTILLATA Ortholog Associated With the Formation of Petaloid Sepals in Double-Flower Eriobotrya japonica (Rosaceae)
Abstract
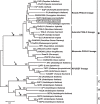
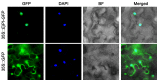
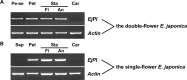
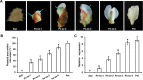
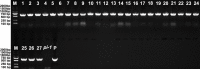
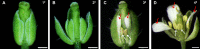
Double-flower Eriobotrya japonica, of which one phenotype is homeotic transformation of sepals into petals, is a new germplasm for revealing the molecular mechanisms underlying the floral organ transformation. Herein, we analyzed the sequence, expression pattern and functional characterization of EjPI, which encoded a B-class floral homeotic protein referred to as PISTILLATA ortholog, from genetically cognate single-flower and double-flower E. japonica. Phylogenetic analysis suggested that the EjPI gene was assigned to the rosids PI/GLO lineage. Analysis of protein sequence alignments showed that EjPI has typical domains of M, I, K, and C, and includes a distinctive PI motif at the C-terminal region. Compared with asterids PI/GLO lineage, the K1 and K3 subdomains of EjPI both contain a single amino acid difference. Subcellular localization of EjPI was determined to be in the nucleus. Expression pattern analysis revealed that EjPI expressed not only in petals, filament, and anther in single-flower E. japonica, but also in petaloid sepals in double-flower E. japonica. Meanwhile, there were high correlation between EjPI transcript level and petaloid area within a sepal. Furthermore, 35S::EjPI transgenic wild-type Arabidopsis caused the homeotic transformation of the first whorl sepals into petaloid sepals. Ectopic expression of EjPI in transgenic pi-1 mutant Arabidopsis rescued normal petals and stamens. These results suggest expression pattern of EjPI is associated with the formation of petaloid sepal. Our study provides the potential application of EjPI for biotechnical engineering to create petaloid sepals or regulate floral organ identity in angiosperms.
Keywords: Eriobotrya japonica; MADS-box gene; PISTILLATA; double-flower; ectopic expression; expression pattern.
Copyright © 2020 Xia, Shi, Chen, Hu, Jing, Wu, Wang, Li, Deng, Guo and Liang.
Figures



References
-
- Bowman J. L., Smyth D. R., Meyerowitz E. M. (1991). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112, 1–20. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

