Puerarin promotes the viability and differentiation of MC3T3-E1 cells by enhancing LC3B-mediated autophagy through downregulation of miR-204
- PMID: 32010248
- PMCID: PMC6966130
- DOI: 10.3892/etm.2019.8291
Puerarin promotes the viability and differentiation of MC3T3-E1 cells by enhancing LC3B-mediated autophagy through downregulation of miR-204
Abstract
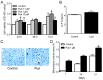
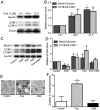
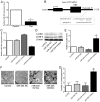
Puerarin is a bioactive substance extracted from Pueraria lobata. It is known to promote the viability, differentiation and mineralization of osteoblasts. However, the molecular mechanisms involved in these activities are not well understood. The present study was conducted with the aim of elucidating the effect of puerarin on osteoblasts and to explore the underlying mechanism. CCK-8 analysis showed that puerarin (0.1, 1 and 10 µM) promoted the viability of osteoblastic MC3T3-E1 cells, with 1 µM of puerarin exhibiting the strongest effect. Moreover, 1 µM puerarin significantly increased the activity of alkaline phosphatase (ALP) and the formation of mineralized nodules in the MC3T3-E1 cells. Treatment with 1 µM puerarin for 72 h led to a significant upregulation in the expression level of microtubule-associated light chain 3 (LC3)B and Beclin1 proteins. This treatment was more effective in promoting LC3B expression than what was observed following treatment with rapamycin (overexpression for autophagy). The bilayer membrane structure of autophagosomes was observed by electron microscopy. Conversely, 3-methyladenine (3-MA, inhibitor of autophagy) reduced the cell viability as well as the activity of alkaline phosphatase (ALP) in MC3T3-E1 cells, although, there was no significant influence on mineralization. Prediction results of the biological information showed that LC3B could be a direct target of microRNA-204 (miR-204). In the present study, the expression level of miR-204 was decreased by puerarin. miR-204 mimics significantly decreased LC3B expression and inhibited auotophagosome formation, while the miR-204 inhibitor had the opposite effects. To conclude, the results of the present study suggest that puerarin promotes the viability and differentiation of MC3T3-E1 cells through autophagy, which is possibly associated with miR-204-regulated LC3B upregulation.
Keywords: LC3B; MC3TC-E1 cells; autophagy; differentiation; miR-204; puerarin; viability.
Copyright: © Feng et al.
Figures

Similar articles
-
Puerarin inhibits TRPM3/miR-204 to promote MC3T3-E1 cells proliferation, differentiation and mineralization.Phytother Res. 2018 Jun;32(6):996-1003. doi: 10.1002/ptr.6034. Epub 2018 Jan 24. Phytother Res. 2018. PMID: 29368357
-
Robo2 promotes osteoblast differentiation and mineralization through autophagy and is activated by parathyroid hormone induction.Ann Anat. 2023 Jun;248:152070. doi: 10.1016/j.aanat.2023.152070. Epub 2023 Feb 17. Ann Anat. 2023. PMID: 36801365
-
Puerarin promotes the proliferation and differentiation of MC3T3-E1 cells via microRNA-106b by targeting receptor activator of nuclear factor-κB ligand.Exp Ther Med. 2018 Jan;15(1):55-60. doi: 10.3892/etm.2017.5405. Epub 2017 Oct 31. Exp Ther Med. 2018. PMID: 29375675 Free PMC article.
-
B-cell lymphoma-2 phosphorylation at Ser70 site-related autophagy mediates puerarin-inhibited the apoptosis of MC3T3-E1 cells during osteoblastogenesis.J Tradit Chin Med. 2024 Feb;44(1):27-34. doi: 10.19852/j.cnki.jtcm.20231024.002. J Tradit Chin Med. 2024. PMID: 38213236 Free PMC article.
-
MicroRNA-590-5p antagonizes the inhibitory effect of high glucose on osteoblast differentiation by suppressing Smad7 in MC3T3-E1 cells.J Int Med Res. 2019 Apr;47(4):1740-1748. doi: 10.1177/0300060519830212. Epub 2019 Feb 26. J Int Med Res. 2019. PMID: 30803283 Free PMC article.
Cited by
-
Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis.Antioxidants (Basel). 2021 Feb 9;10(2):265. doi: 10.3390/antiox10020265. Antioxidants (Basel). 2021. PMID: 33572126 Free PMC article. Review.
-
Does decreased autophagy and dysregulation of LC3A in astrocytes play a role in major depressive disorder?Transl Psychiatry. 2023 Nov 25;13(1):362. doi: 10.1038/s41398-023-02665-2. Transl Psychiatry. 2023. PMID: 38001115 Free PMC article.
References
-
- Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: A review. Chin J Nat Med. 2014;12:407–414. - PubMed
LinkOut - more resources
Full Text Sources
