Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPS-induced sepsis
- PMID: 32010252
- PMCID: PMC6966138
- DOI: 10.3892/etm.2019.8285
Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPS-induced sepsis
Abstract
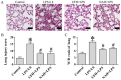
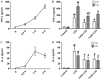
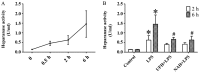
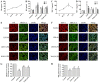
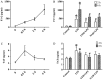
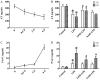
Sepsis commonly progresses to disseminated intravascular coagulation and induces the activation of heparanase (HPA) and the shedding of endothelial glycocalyx constituents, including syndecan-1 (SDC-1) and heparan sulphate (HS). However, the degradation of glycocalyx and its association with coagulation disorders remains undetermined. The present study aimed to evaluate the effect of unfractionated heparin (UFH) and N-acetylheparin (NAH), which is a non-anticoagulant heparin derivative, on endothelial glycocalyx and coagulation function in a lipopolysaccharide (LPS)-induced sepsis rat model, and to compare the differences observed in coagulation function between UFH and NAH. Experimental rats were randomly assigned to four groups: Control; LPS; UFH + LPS; and NAH + LPS. Rats were administered UFH or NAH and subsequently, ~1 min later, administered LPS (10 mg/kg; intravenous). The blood and lung tissues of rats were collected 0.5, 2 and 6 h after LPS injection, and were used for subsequent analysis. The results demonstrated that HPA activity and SDC-1 and HS levels increased, and this increase was associated with inflammatory cytokines and coagulation/fibrinolysis markers in the sepsis rat model. Histopathological examination was performed, and the lung injury score and lung wet/dry ratio indicated that UFH and NAH also significantly improved lung tissue injury. The results of the ELISA analysis demonstrated that UFH and NAH treatment: i) significantly decreased the levels of inflammatory cytokines including tumor necrosis factor-α and interleukin-6; ii) inhibited HPA activity and protected the integrity of the glycocalyx, which was identified by decreased HS and SDC-1 levels; and iii) decreased the levels of prothrombin fragment 1+2, thrombin-antithrombin complex, and plasminogen activator inhibitor-1 and increased the levels of fibrinogen and antithrombin-III. Preconditioning with UFH decreased the plasma activated partial thromboplastin time. These results indicated that UFH and NAH may alleviate sepsis-induced coagulopathy, and this effect may have been due to an inhibition of HPA activity and decrease in the shedding of the endothelial glycocalyx.
Keywords: N-acetylheparin; coagulation; glycocalyx; sepsis; unfractionated heparin.
Copyright: © Huang et al.
Figures
References
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
