Neuropsychological Deficits Chronically Developed after Focal Ischemic Stroke and Beneficial Effects of Pharmacological Hypothermia in the Mouse
- PMID: 32010477
- PMCID: PMC6961763
- DOI: 10.14336/AD.2019.0507
Neuropsychological Deficits Chronically Developed after Focal Ischemic Stroke and Beneficial Effects of Pharmacological Hypothermia in the Mouse
Abstract
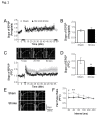
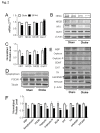
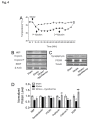
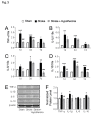
Stroke is a leading cause of human death and disability, with around 30% of stroke patients develop neuropsychological/neuropsychiatric symptoms, such as post-stroke depression (PSD). Basic and translational research on post-stroke psychological disorders is limited. In a focal ischemic stroke mouse model with selective damage to the sensorimotor cortex, sensorimotor deficits develop soon after stroke and spontaneous recovery is observed in 2-4 weeks. We identified that mice subjected to a focal ischemic insult gradually developed depression/anxiety like behaviors 4 to 8 weeks after stroke. Psychological/psychiatric disorders were revealed in multiple behavioral examinations, including the forced swim, tail suspension, sucrose preference, and open field tests. Altered neuronal plasticity such as suppressed long-term potentiation (LTP), reduced BDNF and oxytocin signaling, and disturbed dopamine synthesis/uptake were detected in the prefrontal cortex (PFC) during the chronic phase after stroke. Pharmacological hypothermia induced by the neurotensin receptor 1 (NTR1) agonist HPI-363 was applied as an acute treatment after stroke. A six-hr hypothermia treatment applied 45 min after stroke prevented depression and anxiety like behaviors examined at 6 weeks after stroke, as well as restored BDNF expression and oxytocin signaling. Additionally, hypothermia induced by physical cooling also showed an anti-depression and anti-anxiety effect. The data suggested a delayed beneficial effect of acute hypothermia treatment on chronically developed post-stroke neuropsychological disorders, associated with regulation of synaptic plasticity, neurotrophic factors, dopaminergic activity, and oxytocin signaling in the PFC.
Keywords: BDNF; oxytocin; pharmacological hypothermia; post-stroke depression (PSD); stroke.
Copyright: © 2019 Zhong et al.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest.
Figures

References
-
- Ayerbe L, Ayis S, Wolfe CD, Rudd AG (2013). Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry, 202: 14-21 - PubMed
-
- Kronenberg G, Balkaya M, Prinz V, Gertz K, Ji S, Kirste I, et al. (2012). Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry, 72: 273-281 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
