Cavitation induced by shock wave focusing in eye-like experimental configurations
- PMID: 32010526
- PMCID: PMC6968744
- DOI: 10.1364/BOE.11.000432
Cavitation induced by shock wave focusing in eye-like experimental configurations
Abstract
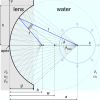
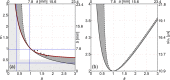
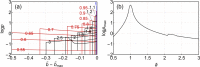
During laser-induced, breakdown-based medical procedures in human eyes such as posterior capsulotomy and vitreolysis, shock waves are emitted from the location of the plasma. A part of these spherically expanding transients is reflected from the concave surface of the corneal epithelium and refocused within the eye. Using a simplified experimental model of the eye, the dominant secondary cavitation clusters were detected by high-speed camera shadowgraphy in the refocusing volume, dislocated from the breakdown position and described by an abridged ray theory. Individual microbubbles were detected in the preheated cone of the incoming laser pulse and radially extending cavitation filaments were generated around the location of the breakdown soon after collapse of the initial bubble. The generation of the secondary cavitation structures due to shock wave focusing can be considered an adverse effect, important in ophthalmology.
© 2019 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors T.P. and R.P. declare that they have filed a patent application PCT/SI2018/050020 for an acoustic diverter for improved safety during ophthalmic laser treatments.
Figures




References
-
- Niemz M. H., Laser-Tissue Interactions: Fundamentals and Applications (Springer, 2007).
-
- Vogel A., Capon M. R. C., Asiyo-Vogel M. N., Birngruber R., “Intraocular Photodisruption with Picosecond and Nanosecond Laser-Pulses - Tissue Effects in Cornea, Lens, and Retina,” Invest. Ophth. Vis. Sci. 35(7), 3032–3044 (1994). - PubMed
-
- Lauterborn W., Vogel A., “Shock wave emission by laser generated bubbles,” in Bubble Dynamics and Shock Waves Delale C. F., ed. (Springer, 2013), pp. 67–103.
-
- Vogel A., Busch S., Parlitz U., “Shock wave emission and cavitation bubble generation by picosecond and nanosecond optical breakdown in water,” J. Acoust. Soc. Am. 100(1), 148–165 (1996).10.1121/1.415878 - DOI
LinkOut - more resources
Full Text Sources
