Irinotecan, topotecan, paclitaxel or docetaxel for second-line treatment of small cell lung cancer: a single-center retrospective study of efficiency comparation and prognosis analysis
- PMID: 32010561
- PMCID: PMC6976368
- DOI: 10.21037/tlcr.2019.10.21
Irinotecan, topotecan, paclitaxel or docetaxel for second-line treatment of small cell lung cancer: a single-center retrospective study of efficiency comparation and prognosis analysis
Abstract
Background: The main aim of this study was to evaluate the efficiency of second-line chemotherapy irinotecan (CPT-11), topotecan (TPT), paclitaxel (PTX) and docetaxel (DTX) in small cell lung cancer (SCLC) patients who have failure to the first-line standard treatment. The secondary aim was to evaluate the independent prognostic factors of patients who received a second line treatment.
Methods: Retrospective analysis of 116 patients who received second-line chemotherapy. Patients were divided into 4 groups according to the therapy they were treated with, which were CPT-11, TPT, PTX and DTX. Progress free survival (PFS), overall survival (OS), objective response rate (ORR) and disease control rate (DCR) were evaluated for each group. Patients' data of clinical character and blood index were collected, and the prognostic factors were assessed both at univariate and multivariate levels.
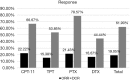
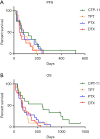
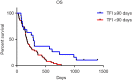
Results: Patients treated with CPT-11 achieved the best median PFS and OS of 91 and 595 days, while the median PFS of TPT, PTX and DTX were 74.5, 81 and 50 days respectively. The median OS of them were 154, 168.5 and 184 days respectively. The survival curves of OS were significantly different (P=0.0069). The reaction to second-line therapy is positively correlate to the reaction to first-line therapy (P=0.012). In the multivariate analysis, treatment free interval (TFI) <90 days, lactate dehydrogenase (LDH) ≥225 U/L, neutrophil-to-lymphocyte ratio (NLR) ≥3.5 were identified as independent risk factors for poor prognosis in second-line SCLC patients.
Conclusions: Second-line chemotherapy with TPT in SCLC patients may provide better overall survival benefits. TFI <90 days, LDH ≥225 U/L and NLR ≥3.5 are independent risk factors for second-line SCLC patients.
Keywords: Second-line therapy; docetaxel; irinotecan; paclitaxel; prognostic factor; small cell lung cancer (SCLC); topotecan.
2019 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
Comment in
-
Treatment strategy for patients with relapsed small-cell lung cancer: past, present and future.Transl Lung Cancer Res. 2020 Apr;9(2):172-179. doi: 10.21037/tlcr.2020.03.10. Transl Lung Cancer Res. 2020. PMID: 32420056 Free PMC article. No abstract available.
-
Second-line therapy for disseminated small-cell lung cancer: optimal management remains to be defined.Transl Lung Cancer Res. 2020 Oct;9(5):1732-1735. doi: 10.21037/tlcr-20-362. Transl Lung Cancer Res. 2020. PMID: 33209596 Free PMC article. No abstract available.
References
-
- Hamilton G, Rath B. Targeting angiogenesis in small cell lung cancer. Transl Cancer Res 2017;6:S522-8. 10.21037/tcr.2017.04.14 - DOI
LinkOut - more resources
Full Text Sources
