Advancement in Determination of Phthalate Metabolites by Gas Chromatography Eliminating Derivatization Step
- PMID: 32010672
- PMCID: PMC6974799
- DOI: 10.3389/fchem.2019.00928
Advancement in Determination of Phthalate Metabolites by Gas Chromatography Eliminating Derivatization Step
Abstract
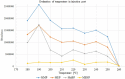
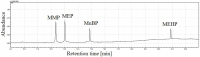
A gas chromatography-mass spectrometry (GC-MS) method to determine polar and thermally unstable phthalate metabolites [monomethyl phthalate-MMP, monoethyl phthalate-MEP, mono-n-butyl phthalate-MnBP, mono-(2-ethylhexyl) phthalate-MEHP] has been developed. This is the first report presenting the separation of monophthalates without derivatization step and any additional equipment or special injection port. Injection parameters (temperature, pressure, time, and volume of injection), chromatographic separation (retention gap, temperature program), and MS detection/identification (working parameters, ion selection) were investigated. Mechanisms and phenomena occurring under different conditions in the GC injector were evaluated and discussed. The limits of detection (LODs) of MMP, MEP, MnBP, MEHP in the protocol were 0.049, 0.036, 0.038, and 0.029 ng (per 2 μL of injection), respectively. The response of the monophthalates was found to be linear in the tested concentration range (for MMP: 0.15-100 ng, MEP and MnBP: 0.11-100 ng, MEHP: 0.087-100 ng per 2 μL) with the coefficient of determination higher than 0.9817 and inter-day precision in the range of 1.4-5.4%. The developed method is fast, easy and repeatable. Moreover, it allows for the elimination of derivatization agents, reduction of toxic waste production and simplification of analytical procedure.
Keywords: analytical method; derivatization; gas chromatography; injection conditions; phthalate metabolites; separation; thermal stability.
Copyright © 2020 Tankiewicz, Olkowska, Berg and Wolska.
Figures



References
-
- Alzaga R., Peña A., Bayona J. M. (2003). Determination of phthalic monoesters in aqueous and urine samples by solid-phase microextraction – diazomethane on-fibre derivatization – gas chromatography – mass spectrometry. J. Sep. Sci. 26, 87–96. 10.1002/jssc.200390020 - DOI
-
- Analytical Methods Committee (1994). Is my calibration linear? Analyst 119, 2363–2366. 10.1039/an9941902363 - DOI
-
- Atkins P. W., De Paula J. (2014). Atkins' Physical Chemistry, 10th Edn. New York, NY: Oxford University Press.
LinkOut - more resources
Full Text Sources
Miscellaneous

