Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study
- PMID: 32010718
- PMCID: PMC6983495
- DOI: 10.1183/23120541.00172-2019
Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study
Abstract
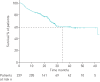
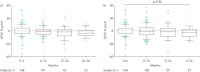
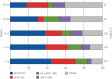
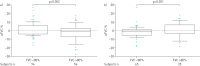
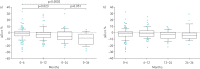
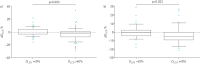
Nintedanib is a tyrosine kinase inhibitor approved for the treatment of idiopathic pulmonary fibrosis (IPF). In a retrospective, real-world study across seven Greek hospitals, we evaluated the effectiveness and safety of nintedanib in routine clinical practice. Patients diagnosed with IPF, as per guideline criteria or multidisciplinary diagnosis, received nintedanib between January 2013 and January 2018. We evaluated 244 patients: mean±sd age 71.8±7.5 years, 79.1% male, 45.1% current smokers and 33.1% ex-smokers at treatment initiation. At baseline, predicted forced vital capacity (FVC) was 73.3±20.7% and predicted diffusing capacity of the lungs for carbon monoxide (D LCO) was 42.6±16.7%. On average, patients spent 23.6±15.0 months on nintedanib. At 3 years, 78 patients had died, equating to a 3-year survival rate of 59.4% (unaffected by treatment discontinuation or dose reduction). FVC% pred and D LCO% pred were largely stable at 3 years, with no significant difference from baseline (FVC 73.3±20.7% pred versus 78±20.1% pred, p=0.074; D LCO 42.6±16.7% pred versus 40.4±18.1% pred, p=0.334). Of the 244 patients, 55.7% reported an adverse event. Gastrointestinal events were the most common (173 (77.2%) out of 224 total events) and 45.0% of patients experienced diarrhoea. Only 32 (13.1%) patients had to permanently discontinue nintedanib due to an adverse event. This real-world study shows a 3-year survival rate of 59.4% and a low discontinuation rate due to adverse events. Our experience is consistent with previous findings in clinical trials of nintedanib in IPF.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: K. Antoniou reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. Conflict of interest: K. Markopoulou reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. Conflict of interest: A. Tzouvelekis reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. In addition, Dr Tzouvelekis has a patent on inhaled or aerosolised delivery of thyroid hormone to the lung as a novel therapeutic agent in fibrotic lung diseases issued to Yale University. Conflict of interest: A. Trachalaki has nothing to disclose. Conflict of interest: E. Vasarmidi has nothing to disclose. Conflict of interest: J. Organtzis has nothing to disclose. Conflict of interest: V. Tzilas has nothing to disclose. Conflict of interest: E. Bouros has nothing to disclose. Conflict of interest: G. Kounti reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. Conflict of interest: C. Rampiadou reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. Conflict of interest: S-C. Kotoulas has nothing to disclose. Conflict of interest: F. Bardaka has nothing to disclose. Conflict of interest: E. Bibaki has nothing to disclose. Conflict of interest: E. Fouka reports travel grants, lecture fees and consultation fees from Boehringer Ingelheim and GlaxoSmithKline, and travel grants and lecture fees from Roche Pharma, AstraZeneca, Novartis, Chiesi, Innovis and Elpen outside the submitted work. Conflict of interest: G. Meletis reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study. Conflict of interest: S. Tryfon has nothing to disclose. Conflict of interest: Z. Daniil reports grants, personal fees, nonfinancial support and other support from BI Hellas during the conduct of the study, and other from Roche outside the submitted work. Conflict of interest: D. Papakosta reports financial support for research, travel grants and lecture fees from Boehringer Ingelheim and Roche, outside the submitted work. Conflict of interest: D. Bouros reports research support, personal fees for lectures and advisory boards, and travel grants from Boehringer Ingelheim, and personal fees for lectures and advisory boards, and travel grants from Roche, outside the submitted work.
Figures
References
LinkOut - more resources
Full Text Sources
