Shape-morphing living composites
- PMID: 32010767
- PMCID: PMC6968942
- DOI: 10.1126/sciadv.aax8582
Shape-morphing living composites
Abstract
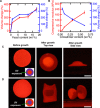
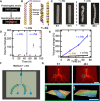
This work establishes a means to exploit genetic networks to create living synthetic composites that change shape in response to specific biochemical or physical stimuli. Baker's yeast embedded in a hydrogel forms a responsive material where cellular proliferation leads to a controllable increase in the composite volume of up to 400%. Genetic manipulation of the yeast enables composites where volume change on exposure to l-histidine is 14× higher than volume change when exposed to d-histidine or other amino acids. By encoding an optogenetic switch into the yeast, spatiotemporally controlled shape change is induced with pulses of dim blue light (2.7 mW/cm2). These living, shape-changing materials may enable sensors or medical devices that respond to highly specific cues found within a biological milieu.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures


References
-
- Wang W., Yao L., Cheng C.-Y., Zhang T., Atsumi H., Wang L., Wang G., Anilionyte O., Steiner H., Ou J., Zhou K., Wawrousek C., Petrecca K., Belcher A. M., Karnik R., Zhao X., Wang D. I. C., Ishii H., Harnessing the hygroscopic and biofluorescent behaviors of genetically tractable microbial cells to design biohybrid wearables. Sci. Adv. 3, e1601984 (2017). - PMC - PubMed
-
- Xu W., Kwok K. S., Gracias D. H., Ultrathin shape change smart materials. Acc. Chem. Res. 51, 436–444 (2018). - PubMed
-
- Hilber W., Stimulus-active polymer actuators for next-generation microfluidic devices. Appl. Phys. A 122, 751 (2016).
-
- Haines C. S., Lima M. D., Li N., Spinks G. M., Foroughi J., Madden J. D. W., Kim S. H., Fang S., Jung de Andrade M., Göktepe F., Göktepe Ö., Mirvakili S. M., Naficy S., Lepró X., Oh J., Kozlov M. E., Kim S. J., Xu X., Swedlove B. J., Wallace G. G., Baughman R. H., Artificial muscles from fishing line and sewing thread. Science 343, 868–872 (2014). - PubMed
-
- Pelrine R., Kornbluh R., Kofod G., High-strain actuator materials based on dielectric elastomers. Adv. Mater. 12, 1223–1225 (2000).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

