Suppression of KIF3A inhibits triple negative breast cancer growth and metastasis by repressing Rb-E2F signaling and epithelial-mesenchymal transition
- PMID: 32011034
- PMCID: PMC7156822
- DOI: 10.1111/cas.14324
Suppression of KIF3A inhibits triple negative breast cancer growth and metastasis by repressing Rb-E2F signaling and epithelial-mesenchymal transition
Abstract
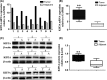
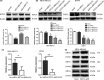
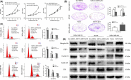
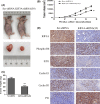
Triple negative breast cancer (TNBC) displays higher heterogeneity, stronger invasiveness, higher risk of metastasis and poorer prognosis compared with major breast cancer subtypes. KIF3A, a member of the kinesin family of motor proteins, serves as a microtubule-directed motor subunit and has been found to regulate early development, ciliogenesis and tumorigenesis. To explore the expression, regulation and mechanism of KIF3A in TNBC, 3 TNBC cell lines, 98 cases of primary TNBC and paired adjacent tissues were examined. Immunohistochemistry, real-time PCR, western blot, flow cytometry, short hairpin RNA (shRNA) interference, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), colony formation techniques, transwell assays, scratch tests, and xenograft mice models were used. We found that KIF3A was overexpressed in TNBC and such high KIF3A expression was also associated with tumor recurrence and lymph node metastasis. Silencing of KIF3A suppressed TNBC cell proliferation by repressing the Rb-E2F signaling pathway and inhibited migration and invasion by repressing epithelial-mesenchymal transition. The tumor size was smaller and the number of lung metastatic nodules was lower in KIF3A depletion MDA-MB-231 cell xenograft mice than in the negative control group. In addition, KIF3A overexpression correlated with chemoresistance. These results suggested that high expression of KIF3A in TNBC was associated with the tumor progression and metastasis.
Keywords: KIF3A; Rb-E2F signaling; Triple negative breast cancer; epithelial-mesenchymal transition; metastasis.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Silencing TRAIP suppresses cell proliferation and migration/invasion of triple negative breast cancer via RB-E2F signaling and EMT.Cancer Gene Ther. 2023 Jan;30(1):74-84. doi: 10.1038/s41417-022-00517-7. Epub 2022 Sep 5. Cancer Gene Ther. 2023. PMID: 36064576 Free PMC article.
-
Targeting PRDX2 to inhibit tumor growth and metastasis in triple-negative breast cancer: the role of FN1 and the PI3K/AKT/SP1 pathway.J Transl Med. 2025 Apr 11;23(1):434. doi: 10.1186/s12967-025-06441-2. J Transl Med. 2025. PMID: 40217291 Free PMC article.
-
Suppression of motor protein KIF3C expression inhibits tumor growth and metastasis in breast cancer by inhibiting TGF-β signaling.Cancer Lett. 2015 Nov 1;368(1):105-114. doi: 10.1016/j.canlet.2015.07.037. Epub 2015 Aug 10. Cancer Lett. 2015. PMID: 26272184
-
MDIG in Breast Cancer Progression and Metastasis.Adv Exp Med Biol. 2024;1465:1-14. doi: 10.1007/978-3-031-66686-5_1. Adv Exp Med Biol. 2024. PMID: 39586990 Review.
-
Mechanisms of RNA alternative splicing dysregulation in triple-negative breast cancer.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Jul 28;49(7):1143-1154. doi: 10.11817/j.issn.1672-7347.2024.240434. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39788502 Free PMC article. Review. Chinese, English.
Cited by
-
Tumor suppressive role of microRNA-139-5p in bone marrow mesenchymal stem cells-derived extracellular vesicles in bladder cancer through regulation of the KIF3A/p21 axis.Cell Death Dis. 2022 Jul 12;13(7):599. doi: 10.1038/s41419-022-04936-0. Cell Death Dis. 2022. PMID: 35821021 Free PMC article.
-
Kinesin family member 3A induces related diseases via wingless-related integration site/β-catenin signaling pathway.Sci Prog. 2023 Jan-Mar;106(1):368504221148340. doi: 10.1177/00368504221148340. Sci Prog. 2023. PMID: 36594221 Free PMC article. Review.
-
tRNA-Derived Fragment tRF-17-79MP9PP Attenuates Cell Invasion and Migration via THBS1/TGF-β1/Smad3 Axis in Breast Cancer.Front Oncol. 2021 Apr 12;11:656078. doi: 10.3389/fonc.2021.656078. eCollection 2021. Front Oncol. 2021. PMID: 33912465 Free PMC article.
-
KIF3A inhibits nasopharyngeal carcinoma proliferation, migration and invasion by interacting with β-catenin to suppress its nuclear accumulation.Am J Cancer Res. 2022 Nov 15;12(11):5226-5240. eCollection 2022. Am J Cancer Res. 2022. PMID: 36504907 Free PMC article.
-
Development and Validation of a Diagnostic Model Based on Hypoxia-Related Genes in Myocardial Infarction.Int J Gen Med. 2023 May 29;16:2111-2123. doi: 10.2147/IJGM.S407759. eCollection 2023. Int J Gen Med. 2023. PMID: 37275329 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Guney EG, Cecener G, Egeli U, Tunca B. Triple negative breast cancer: new therapeutic approaches and BRCA status. APMIS. 2018;126:371‐379. - PubMed
-
- Nemajerova A, Talos F, Moll UM, Petrenko O. Rb function is required for E1A‐induced S‐phase checkpoint activation. Cell Death Differ. 2008;15:1440‐1449. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

