The development and characterization of a long acting anti-thrombotic von Willebrand factor (VWF) aptamer
- PMID: 32011054
- PMCID: PMC7317574
- DOI: 10.1111/jth.14755
The development and characterization of a long acting anti-thrombotic von Willebrand factor (VWF) aptamer
Abstract
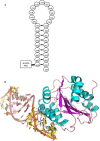
Background: Thrombus formation involves coagulation proteins and platelets. The latter, referred to as platelet-mediated thrombogenesis, is predominant in arterial circulation. Platelet thrombogenesis follows vascular injury when extracellular von Willebrand factor (VWF) binds via its A3 domain to exposed collagen, and the free VWF A1 domain binds to platelet glycoprotein Ib (GPIb).
Objectives: To characterize the antiplatelet/antithrombotic activity of the pegylated VWF antagonist aptamer BT200 and identify the aptamer VWF binding site.
Methods: BT100 is an optimized aptamer synthesized by solid-phase chemistry and pegylated (BT200) by standard conjugation chemistry. The affinity of BT200 for purified human VWF was evaluated as was VWF inhibition in monkey and human plasma. Efficacy of BT200 was assessed in the monkey FeCl3 femoral artery thrombosis model.
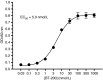
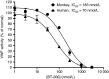
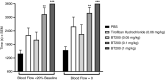
Results: BT200 bound human VWF at an EC50 of 5.0 nmol/L and inhibited VWF A1 domain activity in monkey and human plasma with mean IC50 values of 183 and 70 nmol/L. BT200 administration to cynomolgus monkeys caused a time-dependent and dose-dependent effect on VWF A1 domain activity and inhibited platelet function as measured by collagen adenosine diphosphate closure time in the platelet function analyzer. BT200 demonstrated a bioavailability of ≥77% and exhibited a half-life of >100 hours after subcutaneous injection. The treatment effectively prevented arterial occlusion in an FeCl3 -induced thrombosis model in monkeys.
Conclusions: BT200 has shown promising inhibition of human VWF in vitro and prevented arterial occlusion in non-human primates. These data including a long half-life after subcutaneous injections provide a strong rationale for ongoing clinical development of BT200.
Keywords: aptamers; arterial thrombosis; platelets; primates; von Willebrand factor.
© 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
SZ and JG are employees of Guardian Therapeutics, Inc, PH, WH and BJ consultants. Drs SZ, JG, and BJ also have an equity position with Guardian. SG and DW are employees and ZL is the chairman of Suzhou Ribo Life Science Co.
Figures

References
-
- Sadler JE. von Willebrand factor assembly and secretion. J Thromb Haemost. 2009;7(suppl 1):24‐27. - PubMed
-
- Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428‐2437. - PubMed
-
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395‐424. - PubMed
-
- André P, Hainaud P, Bal dit Sollier C, Garfinkel LI, Caen JP, Drouet LO. Relative involvement of GPIb/IX‐vWF axis and GPIIb/IIIa in thrombus growth at high shear rates in the guinea pig. Arterioscler Thromb Vasc Biol. 1997;17:919‐924. - PubMed
-
- Le Behot A, Gauberti M, Martinez De Lizarrondo S, et al. GpIbalpha‐VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123:3354‐3363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

