Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence
- PMID: 32011232
- PMCID: PMC7064339
- DOI: 10.7554/eLife.48401
Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence
Abstract
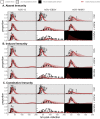
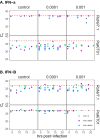
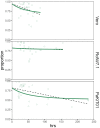
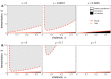
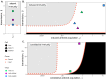
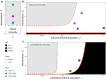
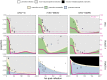
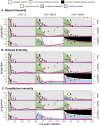
Bats host virulent zoonotic viruses without experiencing disease. A mechanistic understanding of the impact of bats' virus hosting capacities, including uniquely constitutive immune pathways, on cellular-scale viral dynamics is needed to elucidate zoonotic emergence. We carried out virus infectivity assays on bat cell lines expressing induced and constitutive immune phenotypes, then developed a theoretical model of our in vitro system, which we fit to empirical data. Best fit models recapitulated expected immune phenotypes for representative cell lines, supporting robust antiviral defenses in bat cells that correlated with higher estimates for within-host viral propagation rates. In general, heightened immune responses limit pathogen-induced cellular morbidity, which can facilitate the establishment of rapidly-propagating persistent infections within-host. Rapidly-transmitting viruses that have evolved with bat immune systems will likely cause enhanced virulence following emergence into secondary hosts with immune systems that diverge from those unique to bats.
Keywords: Chiroptera; bat; ecology; epidemiology; global health; innate immunity; interferon; within-host model.
Plain language summary
Bats can carry viruses that are deadly to other mammals without themselves showing serious symptoms. In fact, bats are natural reservoirs for viruses that have some of the highest fatality rates of any viruses that people acquire from wild animals – including rabies, Ebola and the SARS coronavirus. Bats have a suite of antiviral defenses that keep the amount of virus in check. For example, some bats have an antiviral immune response called the interferon pathway perpetually switched on. In most other mammals, having such a hyper-vigilant immune response would cause harmful inflammation. Bats, however, have adapted anti-inflammatory traits that protect them from such harm, include the loss of certain genes that normally promote inflammation. However, no one has previously explored how these unique antiviral defenses of bats impact the viruses themselves. Now, Brook et al. have studied this exact question using bat cells grown in the laboratory. The experiments made use of cells from one bat species – the black flying fox – in which the interferon pathway is always on, and another – the Egyptian fruit bat – in which this pathway is only activated during an infection. The bat cells were infected with three different viruses, and then Brook et al. observed how the interferon pathway helped keep the infections in check, before creating a computer model of this response. The experiments and model helped reveal that the bats’ defenses may have a potential downside for other animals, including humans. In both bat species, the strongest antiviral responses were countered by the virus spreading more quickly from cell to cell. This suggests that bat immune defenses may drive the evolution of faster transmitting viruses, and while bats are well protected from the harmful effects of their own prolific viruses, other creatures like humans are not. The findings may help to explain why bats are often the source for viruses that are deadly in humans. Learning more about bats' antiviral defenses and how they drive virus evolution may help scientists develop better ways to predict, prevent or limit the spread of viruses from bats to humans. More studies are needed in bats to help these efforts. In the meantime, the experiments highlight the importance of warning people to avoid direct contact with wild bats.
© 2020, Brook et al.
Conflict of interest statement
CB, MB, KC, AD, CD, AG, BG, MM, MN, LW, Av No competing interests declared
Figures
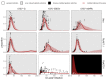

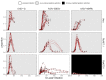
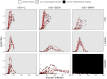
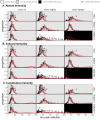


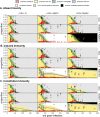
References
-
- Ahn M, Anderson DE, Zhang Q, Tan CW, Lim BL, Luko K, Wen M, Chia WN, Mani S, Wang LC, Ng JHJ, Sobota RM, Dutertre CA, Ginhoux F, Shi ZL, Irving AT, Wang LF. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nature Microbiology. 2019;4:789–799. doi: 10.1038/s41564-019-0371-3. - DOI - PMC - PubMed
-
- Biesold SE, Ritz D, Gloza-Rausch F, Wollny R, Drexler JF, Corman VM, Kalko EK, Oppong S, Drosten C, Müller MA. Type I interferon reaction to viral infection in interferon-competent, immortalized cell lines from the african fruit bat Eidolon helvum. PLOS ONE. 2011;6:e28131. doi: 10.1371/journal.pone.0028131. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- Graduate Research Fellowship/National Science Foundation/International
- NRF2012NRF-CRP001-056/Singapore National Research Foundation Grant/International
- Postdoctoral Fellowship/Adolph C. and Mary Sprague Miller Institute for Basic Research in Science, University of California Berkeley/International
- PREEMPT Program - Cooperative Agreement no. D18AC00031/DARPA/International
- R01 AI129822/AI/NIAID NIH HHS/United States
- #653316/European Union Horizon Grant 2020/International
- NRF2012NRF-CRP001-056/Singapore National Research Foundation/International
- P2C HD047879/HD/NICHD NIH HHS/United States
- R01-AI134824/NH/NIH HHS/United States
- R01 AI134824/AI/NIAID NIH HHS/United States
- #653316/Horizon 2020/International
- RAPID #01KI1723A/German Federal Ministry of Education and Research/International
- NRF2016NRF-NSFC002-013/Singapore National Research Foundation Grant/International
- RAPID #01KI1723A/Bundesministerium für Bildung und Forschung/International
- 1R01AI129822-01/NH/NIH HHS/United States
- DR 772/10-2/Deutsche Forschungsgemeinschaft/International
- NRF2016NRF-NSFC002-013/Singapore National Research Foundation/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

