Opportunities and challenges in using real-world data for health care
- PMID: 32011317
- PMCID: PMC6994109
- DOI: 10.1172/JCI129197
Opportunities and challenges in using real-world data for health care
Abstract
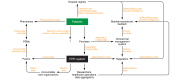
Real-world data (RWD) continue to emerge as a new source of clinical evidence. Although the best-known use case of RWD has been in drug regulation, RWD are being generated and used by many other parties, including biopharmaceutical companies, payors, clinical researchers, providers, and patients. In this Review, we describe 21 potential uses for RWD across the spectrum of health care. We also discuss important challenges and limitations relevant to the translation of these data into evidence.
Conflict of interest statement
Figures
References
-
- FDA. Framework for FDA’s Real-World Evidence Program. 2018. http://www.fda.gov/media/120060/download Accessed December 5, 2019.
-
- Berger M, et al. A framework for regulatory use of real-world evidence. http://healthpolicy.duke.edu/sites/default/files/atoms/files/rwe_white_p... Updated September 13, 2017. Accessed December 5, 2019.
-
- FDA. FDA’s Sentinel Initiative. http://www.fda.gov/safety/fdas-sentinel-initiative Updated October 18, 2019. Accessed December 5, 2019.
-
- FDA. MedWatch: The FDA Safety Information and Adverse Event Reporting Program. http://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-ev... Updated December 2, 2019. Accessed December 5, 2019.

