Assessment of C-Reactive Protein Diagnostic Test Accuracy for Late-Onset Infection in Newborn Infants: A Systematic Review and Meta-analysis
- PMID: 32011640
- PMCID: PMC7042944
- DOI: 10.1001/jamapediatrics.2019.5669
Assessment of C-Reactive Protein Diagnostic Test Accuracy for Late-Onset Infection in Newborn Infants: A Systematic Review and Meta-analysis
Erratum in
-
Typographical Errors and Omitted Reference to Related Meta-analysis.JAMA Pediatr. 2020 Jun 1;174(6):625. doi: 10.1001/jamapediatrics.2020.0950. JAMA Pediatr. 2020. PMID: 32364579 Free PMC article. No abstract available.
Abstract
Importance: Rapid and accurate diagnosis of late-onset infection in newborn infants could inform treatment decisions and avoid unnecessary administration of antibiotics.
Objective: To compare the accuracy of serum C-reactive protein (CRP) with that of microbiological blood culture for diagnosing late-onset infection in newborns.
Data sources: MEDLINE (1946-2019), Embase (1946-2019), and Science Citation Index (1900-2019) databases were searched for references (any language). The MeSH search terms included were "exp infant, newborn/" or "premature birth/" plus free text synonyms; and "C-reactive protein/" plus free text synonyms; and "exp sepsis/" or "exp bacterial infections/" plus free text synonyms. The proceedings from relevant conferences and references of identified papers were scrutinized. Authors were contacted to request missing data.
Study selection: Cohort and cross-sectional studies were included that compared the accuracy of serum CRP levels with microbiological culture results to diagnose late-onset (>72 hours after birth) infection in newborns (any gestational age) hospitalized after birth. Two reviewers assessed study eligibility. Among 10 394 records, 148 studies were assessed as full texts.
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline extension for Diagnostic Test Accuracy (DTA) reviews was followed. Two reviewers assessed the method quality of each study using guidance from the Cochrane Screening and Diagnostic Test Methods Group (adapted from the Quality Assessment of Diagnostic Accuracy Studies 2).
Main outcomes and measures: The primary meta-analysis outcome was diagnostic test accuracy of serum CRP level taken at initial investigation of an infant with suspected late-onset infection. The median specificity (proportion of true-negative results) and calculated pooled sensitivity (proportion of true-positive results) were determined by generating hierarchical summary receiver characteristic operating curves.
Results: In total, 22 studies with 2255 infants were included (sample size range, 11-590 infants). Participants in most studies were preterm (<37 weeks) or very low-birth weight (<1500 g) infants. Two studies additionally enrolled infants born at term. Most studies (16) used a prespecified CRP level cutoff for a "positive" index test (5-10 mg/L), and most studies (17) used the culture of a pathogenic microorganism from blood as the reference standard. Risk of bias was low with independent assessment of index and reference tests. At median specificity (0.74), pooled sensitivity was 0.62 (95% CI, 0.50-0.72). Adding serum CRP level to the assessment of an infant with a 40% pretest probability of late-onset infection (the median for the included studies) generated posttest probabilities of 26% for a negative test result and 61% for a positive test result.
Conclusions and relevance: The findings suggest that determination of serum CRP level at initial evaluation of an infant with suspected late-onset infection is unlikely to aid early diagnosis or to select infants to undergo further investigation or treatment with antimicrobial therapy or other interventions.
Conflict of interest statement
Figures
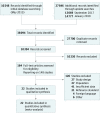

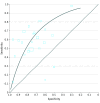

Comment in
-
C-Reactive Protein Testing in Late-Onset Neonatal Sepsis: Hazardous Waste.JAMA Pediatr. 2020 Mar 1;174(3):235-236. doi: 10.1001/jamapediatrics.2019.5684. JAMA Pediatr. 2020. PMID: 32011645 No abstract available.
-
Role of C-Reactive Protein for Late-Onset Neonatal Sepsis.JAMA Pediatr. 2021 Jan 1;175(1):100-101. doi: 10.1001/jamapediatrics.2020.2126. JAMA Pediatr. 2021. PMID: 32804193 No abstract available.
-
Role of C-Reactive Protein for Late-Onset Neonatal Sepsis.JAMA Pediatr. 2021 Jan 1;175(1):101-102. doi: 10.1001/jamapediatrics.2020.2129. JAMA Pediatr. 2021. PMID: 32804199 No abstract available.
References
-
- Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS; Canadian Neonatal Network . Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks’ gestation. Am J Perinatol. 2015;32(7):675-682. - PubMed
-
- Bassler D, Stoll BJ, Schmidt B, et al. ; Trial of Indomethacin Prophylaxis in Preterms Investigators . Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313-318. doi: 10.1542/peds.2008-0377 - DOI - PMC - PubMed
-
- Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357-2365. doi: 10.1001/jama.292.19.2357 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

