Full-length NF-κB repressing factor contains an XRN2 binding domain
- PMID: 32011671
- PMCID: PMC7054742
- DOI: 10.1042/BCJ20190733
Full-length NF-κB repressing factor contains an XRN2 binding domain
Abstract
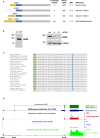
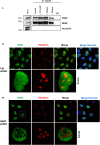
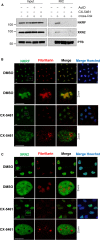
NF-κB repressing factor (NKRF) was recently identified as an RNA binding protein that together with its associated proteins, the 5'-3' exonuclease XRN2 and the helicase DHX15, is required to process the precursor ribosomal RNA. XRN2 is a multi-functional ribonuclease that is also involved in processing mRNAs, tRNAs and lncRNAs. The activity and stability of XRN2 are controlled by its binding partners, PAXT-1, CDKN2AIP and CDKN2AIPNL. In each case, these proteins interact with XRN2 via an XRN2 binding domain (XTBD), the structure and mode of action of which is highly conserved. Rather surprisingly, although NKRF interacts directly with XRN2, it was not predicted to contain such a domain, and NKRF's interaction with XRN2 was therefore unexplained. We have identified an alternative upstream AUG start codon within the transcript that encodes NKRF and demonstrate that the full-length form of NKRF contains an XTBD that is conserved across species. Our data suggest that NKRF is tethered in the nucleolus by binding directly to rRNA and that the XTBD in the N-terminal extension of NKRF is essential for the retention of XRN2 in this sub-organelle. Thus, we propose NKRF regulates the early steps of pre-rRNA processing during ribosome biogenesis by controlling the spatial distribution of XRN2 and our data provide further support for the XTBD as an XRN2 interacting motif.
Keywords: RNA-binding proteins; XRN2; XTBD; protein synthesis; ribosome biogenesis.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

