Interstitial Lung Disease in Relatives of Patients with Pulmonary Fibrosis
- PMID: 32011908
- PMCID: PMC7233344
- DOI: 10.1164/rccm.201908-1571OC
Interstitial Lung Disease in Relatives of Patients with Pulmonary Fibrosis
Abstract
Rationale: Although relatives of patients with familial pulmonary fibrosis (FPF) are at an increased risk for interstitial lung disease (ILD), the risk among relatives of sporadic idiopathic pulmonary fibrosis (IPF) is not known.Objectives: To identify the prevalence of interstitial lung abnormalities (ILA) and ILD among relatives of patients with FPF and sporadic IPF.Methods: Undiagnosed first-degree relatives of patients with pulmonary fibrosis (PF) consented to participate in a screening study that included the completion of questionnaires, pulmonary function testing, chest computed tomography, a blood sample collection for immunophenotyping, telomere length assessments, and genetic testing.Measurements and Main Results: Of the 105 relatives in the study, 33 (31%) had ILA, whereas 72 (69%) were either indeterminate or had no ILA. Of the 33 relatives with ILA, 19 (58%) had further evidence for ILD (defined by the combination of imaging findings and pulmonary function testing decrements). There was no evidence in multivariable analyses that the prevalence of either ILA or ILD differed between the 46 relatives with FPF and the 59 relatives with sporadic IPF. Relatives with decrements in either total lung or diffusion capacity had a greater than 9-fold increase in their odds of having ILA (odds ratio, 9.6; 95% confidence interval, 3.1-29.8; P < 0.001).Conclusions: An undiagnosed form of ILD may be present in greater than 1 in 6 older first-degree relatives of patients with PF. First-degree relatives of patients with both familial and sporadic IPF appear to be at similar risk. Our findings suggest that screening for PF in relatives might be warranted.
Keywords: familial pulmonary fibrosis; idiopathic pulmonary fibrosis; interstitial lung abnormalities; interstitial lung disease; screening.
Figures
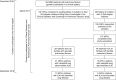
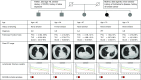

Comment in
References
-
- Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. IPF Study Group. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–967. - PubMed
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. - PubMed
-
- García-Sancho C, Buendía-Roldán I, Fernández-Plata MR, Navarro C, Pérez-Padilla R, Vargas MH, et al. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105:1902–1907. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

