Prospective Evaluation of HIV Testing Technologies in a Clinical Setting: Protocol for Project DETECT
- PMID: 32012115
- PMCID: PMC7011122
- DOI: 10.2196/16332
Prospective Evaluation of HIV Testing Technologies in a Clinical Setting: Protocol for Project DETECT
Abstract
Background: HIV testing guidelines provided by the Centers for Disease Control and Prevention (CDC) are continually changing to reflect advancements in new testing technology. Evaluation of existing and new point-of-care (POC) HIV tests is crucial to inform testing guidelines and provide information to clinicians and other HIV test providers. Characterizing the performance of POC HIV tests using unprocessed specimens can provide estimates for the window period of detection, or the time from HIV acquisition to test positivity, which allows clinicians and other HIV providers to select the appropriate POC HIV tests for persons who may be recently infected with HIV.
Objective: This paper describes the protocols and procedures used to evaluate the performance of the newest POC tests and determine their sensitivity during early HIV infection.
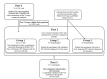
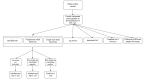
Methods: Project DETECT is a CDC-funded study that is evaluating POC HIV test performance. Part 1 is a cross-sectional, retrospective study comparing behavioral characteristics and HIV prevalence of the overall population of the Public Health-Seattle & King County (PHSKC) Sexually Transmitted Disease (STD) Clinic to Project DETECT participants enrolled in part 2. Part 2 is a cross-sectional, prospective study evaluating POC HIV tests in real time using unprocessed whole blood and oral fluid specimens. A POC nucleic acid test (NAT) was added to the panel of HIV tests in June 2018. Part 3 is a longitudinal, prospective study evaluating seroconversion sensitivity of POC HIV tests through serial follow-up testing. For comparison, HIV-1 RNA and HIV-1/HIV-2 antigen/antibody tests are also performed for participants enrolled in part 2 or 3. A behavioral survey that collects information about demographics, history of HIV testing, STD history, symptoms of acute HIV infection, substance use, sexual behaviors in the aggregate and with recent partners, and use of pre-exposure prophylaxis and antiretroviral therapy is completed at each part 2 or 3 visit.
Results: Between September 2015 and March 2019, there were 14,990 Project DETECT-eligible visits (part 1) to the PHSKC STD Clinic resulting in 1819 part 2 Project DETECT study visits. The longitudinal study within Project DETECT (part 3) enrolled 27 participants with discordant POC test results from their part 2 visit, and 10 (37%) were followed until they had fully seroconverted with concordant positive POC test results. Behavioral survey data and HIV test results, sensitivity, and specificity will be presented elsewhere.
Conclusions: Studies such as Project DETECT are critical for evaluating POC HIV test devices as well as describing characteristics of persons at risk for HIV acquisition in the United States. HIV tests in development, including POC NATs, will provide new opportunities for HIV testing programs.
International registered report identifier (irrid): RR1-10.2196/16332.
Keywords: HIV testing; acute HIV infection; nucleic acid tests; point-of-care tests.
©Joanne D Stekler, Lauren R Violette, Hollie A Clark, Sarah J McDougal, Lisa A Niemann, David A Katz, Pollyanna R Chavez, Laura G Wesolowski, Steven F Ethridge, Vanessa M McMahan, Andy Cornelius-Hudson, Kevin P Delaney. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 27.01.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Centers for Disease Control and Prevention Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm—United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013 Jun 21;62(24):489–494. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6224a2.htm - PMC - PubMed
-
- White DAE, Giordano TP, Pasalar S, Jacobson KR, Glick NR, Sha BE, Mammen PE, Hunt BR, Todorovic T, Moreno-Walton L, Adomolga V, Feaster DJ, Branson BM. Acute HIV discovered during routine HIV screening with HIV antigen-antibody combination tests in 9 US emergency departments. Ann Emerg Med. 2018 Jul;72(1):29–40. doi: 10.1016/j.annemergmed.2017.11.027. - DOI - PubMed
-
- Peters PJ, Westheimer E, Cohen S, Hightow-Weidman LB, Moss N, Tsoi B, Hall L, Fann C, Daskalakis DC, Beagle S, Patel P, Radix A, Foust E, Kohn RP, Marmorino J, Pandori M, Fu J, Samandari T, Gay CL. Screening yield of HIV antigen/antibody combination and pooled HIV RNA testing for acute HIV infection in a high-prevalence population. JAMA. 2016 Feb 16;315(7):682–690. doi: 10.1001/jama.2016.0286. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials