Loncastuximab tesirine, an anti-CD19 antibody-drug conjugate, in relapsed/refractory B-cell acute lymphoblastic leukemia
- PMID: 32012214
- PMCID: PMC7013258
- DOI: 10.1182/bloodadvances.2019000767
Loncastuximab tesirine, an anti-CD19 antibody-drug conjugate, in relapsed/refractory B-cell acute lymphoblastic leukemia
Abstract
Relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) remains a therapeutic challenge. Loncastuximab tesirine is an antibody-drug conjugate against CD19, an antigen expressed in many B-cell malignancies. This open-label, single-arm, dose-escalation, dose-expansion study assessed the safety, tolerability, pharmacokinetics (PKs), immunogenicity, and preliminary clinical activity of loncastuximab tesirine in adults with R/R B-ALL. A total of 35 patients were enrolled, with a median age of 55 years (range, 20-80) and a median of 3 prior therapies (range, 1-15). All patients received at least 1 IV infusion of loncastuximab tesirine at 15 to 150 μg/kg once every 3 weeks (Q3W; n = 30) or 50 μg/kg IV weekly (n = 5). Common treatment-emergent adverse events (TEAEs) were nausea (42.9%), febrile neutropenia (37.1%), and reversible liver test abnormalities. Grade ≥3 TEAEs were reported in 85.7% patients, most commonly febrile neutropenia and other hematologic abnormalities and reversible liver test abnormalities. There were no treatment-related deaths. Four patients (11.4%) had grade 2 infusion-related reactions, and 1 patient (150 μg/kg Q3W) had a dose-limiting toxicity of hyperbilirubinemia that resolved within 6 days without further action. The maximum tolerated dose was not reached. Three patients achieved complete responses, 1 each at 30, 120, and 150 μg/kg Q3W. PK studies showed marked interpatient variability, with target-mediated drug disposition seeming to contribute to time- and dose-dependent disposition. No clinically relevant anti-drug-antibody formation occurred. The trial was terminated in the dose-escalation phase because of slow accrual. This trial was registered at www.clinicaltrials.gov as NCT02669264.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: N.J. has received research funding and honoraria from ADC Therapeutics SA, research funding and honoraria from Servier, research funding and honoraria from Pfizer, research funding and honoraria from Precision Biosciences, research funding from Cellectis, research funding from Incyte, and research funding and honoraria from Adaptive Biotechnologies. A.Z. has received research funding (institutional) from Celgene, Acceleron, AbbVie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics and has also had a consultancy agreement with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, and Takeda (none of these relationships were related to the development of this manuscript). L.H. has received institutional research funding from ADC Therapeutics SA. N.J., H.K., and W.S. are advisory board members for ADC Therapeutics SA. J.F., D.U., G.C., X.Z., Y.Q., and K.H. are employees of ADC Therapeutics with stock options. B.B. is an advisory board member for Novartis and Astellas. The remaining authors declare no competing financial interests.
Figures
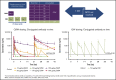
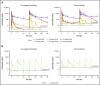
References
-
- Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C; ESMO Guidelines Committee . Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69-v82. - PubMed
-
- BLINCYTO prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125557s008lbl.pdf. Accessed 26 April 2019.
-
- BESPONSA prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf. Accessed 26 April 2019.
-
- Kymriah prescribing information. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapy.... Accessed 26 April 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

