Neuropeptide Y Enhances Progerin Clearance and Ameliorates the Senescent Phenotype of Human Hutchinson-Gilford Progeria Syndrome Cells
- PMID: 32012215
- PMCID: PMC7243588
- DOI: 10.1093/gerona/glz280
Neuropeptide Y Enhances Progerin Clearance and Ameliorates the Senescent Phenotype of Human Hutchinson-Gilford Progeria Syndrome Cells
Abstract
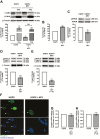
Hutchinson-Gilford progeria syndrome (HGPS, or classical progeria) is a rare genetic disorder, characterized by premature aging, and caused by a de novo point mutation (C608G) within the lamin A/C gene (LMNA), producing an abnormal lamin A protein, termed progerin. Accumulation of progerin causes nuclear abnormalities and cell cycle arrest ultimately leading to cellular senescence. Autophagy impairment is a hallmark of cellular aging, and the rescue of this proteostasis mechanism delays aging progression in HGPS cells. We have previously shown that the endogenous Neuropeptide Y (NPY) increases autophagy in hypothalamus, a brain area already identified as a central regulator of whole-body aging. We also showed that NPY mediates caloric restriction-induced autophagy. These results are in accordance with other studies suggesting that NPY may act as a caloric restriction mimetic and plays a role as a lifespan and aging regulator. The aim of the present study was, therefore, to investigate if NPY could delay HGPS premature aging phenotype. Herein, we report that NPY increases autophagic flux and progerin clearance in primary cultures of human dermal fibroblasts from HGPS patients. NPY also rescues nuclear morphology and decreases the number of dysmorphic nuclei, a hallmark of HGPS cells. In addition, NPY decreases other hallmarks of aging as DNA damage and cellular senescence. Altogether, these results show that NPY rescues several hallmarks of cellular aging in HGPS cells, suggesting that NPY can be considered a promising strategy to delay or block the premature aging of HGPS.
Keywords: Autophagy; Caloric restriction mimetic; Cellular senescence; Human aging.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Gerontological Society of America.
Figures


References
-
- Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249–264. doi: 10.1016/B978-0-444-62702-5.00018-4 - PubMed
-
- De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

