Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole
- PMID: 32012658
- PMCID: PMC7157581
- DOI: 10.3390/tropicalmed5010017
Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole
Abstract
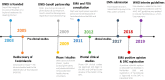
Human African Trypanosomiasis (HAT or sleeping sickness) is a life-threatening neglected tropical disease that is endemic in 36 sub-Saharan African countries. Until recently, treatment options were limited and hampered by unsatisfactory efficacy, toxicity, and long and cumbersome administration regimens, compounded by infrastructure inadequacies in the remote rural regions worst affected by the disease. Increased funding and awareness of HAT over the past two decades has led to a steady decline in reported cases (<1000 in 2018). Recent drug development strategies have resulted in development of the first all-oral treatment for HAT, fexinidazole. Fexinidazole received European Medicines Agency positive scientific opinion in 2018 and is now incorporated into the WHO interim guidelines as one of the first-line treatments for HAT, allowing lumbar puncture to become non-systematic. Here, we highlight the role of global collaborations in the effort to control HAT and develop new treatments. The long-standing collaboration between the WHO, Sanofi and the Drugs for Neglected Diseases initiative (Geneva, Switzerland) was instrumental for achieving the control and treatment development goals in HAT, whilst at the same time ensuring that efforts were led by national authorities and control programs to leave a legacy of highly trained healthcare workers and improved research and health infrastructure.
Keywords: T. b. gambiense; T. b. rhodesiense; elimination; fexinidazole; g-HAT; human African trypanosomiasis; neglected tropical diseases; r-HAT; sleeping sickness.
Conflict of interest statement
H.H., V.L., P.N., and L.K. are all employees of Sanofi. N.S. is an employee of DND
Figures



References
-
- Drugs for Neglected Diseases Initiative About Sleeping Sickness. [(accessed on 11 November 2019)]; Available online: https://www.dndi.org/diseases-projects/hat/
-
- World Health Organization WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis. [(accessed on 17 December 2019)];2019 Available online: https://www.who.int/trypanosomiasis_african/resources/9789241550567/en/ - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

