Prenatal and Peripartum Exposure to Antibiotics and Cesarean Section Delivery Are Associated with Differences in Diversity and Composition of the Infant Meconium Microbiome
- PMID: 32012716
- PMCID: PMC7074690
- DOI: 10.3390/microorganisms8020179
Prenatal and Peripartum Exposure to Antibiotics and Cesarean Section Delivery Are Associated with Differences in Diversity and Composition of the Infant Meconium Microbiome
Abstract
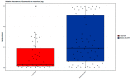
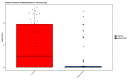
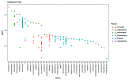
The meconium microbiome may provide insight into intrauterine and peripartum exposures and the very earliest intestinal pioneering microbes. Prenatal antibiotics have been associated with later obesity in children, which is thought to be driven by microbiome dependent mechanisms. However, there is little data regarding associations of prenatal or peripartum antibiotic exposure, with or without cesarean section (CS), with the features of the meconium microbiome. In this study, 16S ribosomal RNA gene sequencing was performed on bacterial DNA of meconium samples from 105 infants in a birth cohort study. After multivariable adjustment, delivery mode (p = 0.044), prenatal antibiotic use (p = 0.005) and peripartum antibiotic use (p < 0.001) were associated with beta diversity of the infant meconium microbiome. CS (vs. vaginal delivery) and peripartum antibiotics were also associated with greater alpha diversity of the meconium microbiome (Shannon and Simpson, p < 0.05). Meconium from infants born by CS (vs. vaginal delivery) had lower relative abundance of the genus Escherichia (p < 0.001). Prenatal antibiotic use and peripartum antibiotic use (both in the overall analytic sample and when restricting to vaginally delivered infants) were associated with differential abundance of several bacterial taxa in the meconium. Bacterial taxa in the meconium microbiome were also differentially associated with infant excess weight at 12 months of age, however, sample size was limited for this comparison. In conclusion, prenatal and peripartum antibiotic use along with CS delivery were associated with differences in the diversity and composition of the meconium microbiome. Whether or not these differences in the meconium microbiome portend risk for long-term health outcomes warrants further exploration.
Keywords: antibiotics; delivery mode; infant; microbiome; neonate; pediatrics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

