A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells
- PMID: 32012717
- PMCID: PMC7072213
- DOI: 10.3390/cancers12020298
A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells
Abstract
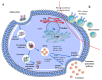
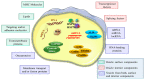
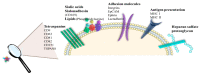
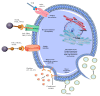
Critical processes such as growth, invasion, and metastasis of cancer cells are sustained via bidirectional cell-to-cell communication in tissue complex environments. Such communication involves the secretion of soluble factors by stromal cells and/or cancer cells within the tumor microenvironment (TME). Both stromal and cancer cells have been shown to export bilayer nanoparticles: encapsulated regulatory molecules that contribute to cell-to-cell communication. These nanoparticles are known as extracellular vesicles (EVs) being classified into exosomes, microvesicles, and apoptotic bodies. EVs carry a vast repertoire of molecules such as oncoproteins and oncopeptides, DNA fragments from parental to target cells, RNA species (mRNAs, microRNAs, and long non-coding RNA), and lipids, initiating phenotypic changes in TME. According to their specific cargo, EVs have crucial roles in several early and late processes associated with tumor development and metastasis. Emerging evidence suggests that EVs are being investigated for their implication in early cancer detection, monitoring cancer progression and chemotherapeutic response, and more relevant, the development of novel targeted therapeutics. In this study, we provide a comprehensive understanding of the biophysical properties and physiological functions of EVs, their implications in TME, and highlight the applicability of EVs for the development of cancer diagnostics and therapeutics.
Keywords: biogenesis; cancer; clinical implications; extracellular vesicles; function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thery C., Regnault A., Garin J., Wolfers J., Zitvogel L., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

