Environmental Enrichment: Disentangling the Influence of Novelty, Social, and Physical Activity on Cerebral Amyloid Angiopathy in a Transgenic Mouse Model
- PMID: 32012921
- PMCID: PMC7038188
- DOI: 10.3390/ijms21030843
Environmental Enrichment: Disentangling the Influence of Novelty, Social, and Physical Activity on Cerebral Amyloid Angiopathy in a Transgenic Mouse Model
Abstract
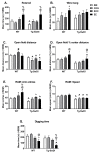
Cerebral amyloid angiopathy (CAA) is the deposition of amyloid protein in the cerebral vasculature, a common feature in both aging and Alzheimer's disease (AD). However, the effects of environmental factors, particularly cognitive stimulation, social stimulation, and physical activity, on CAA pathology are poorly understood. These factors, delivered in the form of the environmental enrichment (EE) paradigm in rodents, have been shown to have beneficial effects on the brain and behavior in healthy aging and AD models. However, the relative importance of these subcomponents on CAA pathology has not been investigated. Therefore, we assessed the effects of EE, social enrichment (SOC), and cognitive enrichment (COG) compared to a control group that was single housed without enrichment (SIN) from 4 to 8 months of age in wild-type mice (WT) and Tg-SwDI mice, a transgenic mouse model of CAA that exhibits cognitive/behavioral deficits. The results show that individual facets of enrichment can affect an animal model of CAA, though the SOC and combined EE conditions are generally the most effective at producing physiological, cognitive/behavioral, and neuropathological changes, adding to a growing literature supporting the benefits of lifestyle interventions.
Keywords: Alzheimer’s disease; cerebral amyloid angiopathy; enriched environment; exercise; reserve; resilience.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Holland C.M., Smith E.E., Csapo I., Gurol M.E., Brylka D.A., Killiany R.J., Blacker D., Albert M.S., Guttmann C.R., Greenberg S.M. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

