RPGR-Associated Dystrophies: Clinical, Genetic, and Histopathological Features
- PMID: 32012938
- PMCID: PMC7038140
- DOI: 10.3390/ijms21030835
RPGR-Associated Dystrophies: Clinical, Genetic, and Histopathological Features
Abstract
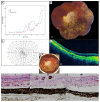
This study describes the clinical, genetic, and histopathological features in patients with RPGR-associated retinal dystrophies. Nine male patients from eight unrelated families underwent a comprehensive ophthalmic examination. Additionally, the histopathology of the right eye from a patient with an end-stage cone-rod-dystrophy (CRD)/sector retinitis pigmentosa (RP) phenotype was examined. All RPGR mutations causing a CRD phenotype were situated in exon ORF15. The mean best-corrected visual acuity (BCVA, decimals) was 0.58 (standard deviation (SD)): 0.34; range: 0.05-1.13); and the mean spherical refractive error was -4.1 D (SD: 2.11; range: -1.38 to -8.19). Hyperautofluorescent rings were observed in six patients. Full-field electroretinography responses were absent in all patients. The visual field defects ranged from peripheral constriction to central islands. The mean macular sensitivity on microperimetry was 11.6 dB (SD: 7.8; range: 1.6-24.4) and correlated significantly with BCVA (r = 0.907; p = 0.001). A histological examination of the donor eye showed disruption of retinal topology and stratification, with a more severe loss found in the peripheral regions. Reactive gliosis was seen in the inner layers of all regions. Our study demonstrates the highly variable phenotype found in RPGR-associated retinal dystrophies. Therapies should be applied at the earliest signs of photoreceptor degeneration, prior to the remodeling of the inner retina.
Keywords: Retinitis Pigmentosa GTPase Regulator (RPGR), cone-rod dystrophy; genotype-phenotype; histopathology; natural history; retinal dystrophies; retinitis pigmentosa.
Conflict of interest statement
The authors declare no conflict of interest
Figures



Similar articles
-
Detailed comparison of phenotype between male patients carrying variants in exons 1-14 and ORF15 of RPGR.Exp Eye Res. 2020 Sep;198:108147. doi: 10.1016/j.exer.2020.108147. Epub 2020 Jul 21. Exp Eye Res. 2020. PMID: 32702353
-
Macular Dystrophy and Cone-Rod Dystrophy Caused by Mutations in the RP1 Gene: Extending the RP1 Disease Spectrum.Invest Ophthalmol Vis Sci. 2019 Mar 1;60(4):1192-1203. doi: 10.1167/iovs.18-26084. Invest Ophthalmol Vis Sci. 2019. PMID: 30913292
-
Discordant phenotypes in fraternal twins having an identical mutation in exon ORF15 of the RPGR gene.Arch Ophthalmol. 2008 Mar;126(3):379-84. doi: 10.1001/archophthalmol.2007.72. Arch Ophthalmol. 2008. PMID: 18332319
-
Clinical applications of microperimetry in RPGR-related retinitis pigmentosa: a review.Acta Ophthalmol. 2021 Dec;99(8):819-825. doi: 10.1111/aos.14816. Epub 2021 Mar 29. Acta Ophthalmol. 2021. PMID: 33783139 Review.
-
Mutations of RPGR in X-linked retinitis pigmentosa (RP3).Hum Mutat. 2002 May;19(5):486-500. doi: 10.1002/humu.10057. Hum Mutat. 2002. PMID: 11968081 Review.
Cited by
-
Genotype-Phenotype Analysis of RPGR Variations: Reporting of 62 Chinese Families and a Literature Review.Front Genet. 2021 Jun 23;12:600210. doi: 10.3389/fgene.2021.600210. eCollection 2021. Front Genet. 2021. PMID: 34745198 Free PMC article.
-
Impaired glutamylation of RPGRORF15 underlies the cone-dominated phenotype associated with truncating distal ORF15 variants.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2208707119. doi: 10.1073/pnas.2208707119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36445968 Free PMC article.
-
CRYAA and GJA8 promote visual development after whisker tactile deprivation.Heliyon. 2023 Feb 23;9(3):e13897. doi: 10.1016/j.heliyon.2023.e13897. eCollection 2023 Mar. Heliyon. 2023. PMID: 36915480 Free PMC article.
-
Retinitis Pigmentosa: Current Clinical Management and Emerging Therapies.Int J Mol Sci. 2023 Apr 19;24(8):7481. doi: 10.3390/ijms24087481. Int J Mol Sci. 2023. PMID: 37108642 Free PMC article. Review.
-
Sector Retinitis Pigmentosa: Extending the Molecular Genetics Basis and Elucidating the Natural History.Am J Ophthalmol. 2021 Jan;221:299-310. doi: 10.1016/j.ajo.2020.08.004. Epub 2020 Aug 12. Am J Ophthalmol. 2021. PMID: 32795431 Free PMC article.
References
-
- Talib M., van Schooneveld M.J., Thiadens A.A., Fiocco M., Wijnholds J., Florijn R.J., Schalij-Delfos N.E., van Genderen M.M., Putter H., Cremers F.P.M., et al. Clinical and Genetic characteristics of Male patients with RPGR-associated Retinal Dystrophies: A long-Term Follow-up Study. RETINA. 2019;39:1186–1199. doi: 10.1097/IAE.0000000000002125. - DOI - PubMed
-
- Tee J.J.L., Yang Y., Kalitzeos A., Webster A., Bainbridge J., Weleber R.G., Michaelides M. Characterization of Visual Function, Interocular Variability and Progression Using Static Perimetry–Derived Metrics in RPGR-Associated Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018;59:2422–2436. doi: 10.1167/iovs.17-23739. - DOI - PMC - PubMed
-
- Bader I., Brandau O., Achatz H., Apfelstedt-Sylla E., Hergersberg M., Lorenz B., Wissinger B., Wittwer B.r., Rudolph G.n., Meindl A., et al. X-linked Retinitis Pigmentosa: RPGR Mutations in Most Families with Definite X Linkage and Clustering of Mutations in a Short Sequence Stretch of Exon ORF15. Investig. Ophthalmol. Vis. Sci. 2003;44:1458–1463. doi: 10.1167/iovs.02-0605. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

