Introduction of Mercaptoethyl at Sorafenib Pyridine-2-Amide Motif as a Potentially Effective Chain to Further get Sorafenib-PEG-DGL
- PMID: 32013003
- PMCID: PMC7037283
- DOI: 10.3390/molecules25030573
Introduction of Mercaptoethyl at Sorafenib Pyridine-2-Amide Motif as a Potentially Effective Chain to Further get Sorafenib-PEG-DGL
Abstract
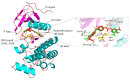
The crystal structure of the sorafenib and B-RAF complex indicates that the binding cavity occupied by the pyridine-2-carboxamide in sorafenib has a large variable space, making it a reasonable modification site. In order to identify novel compounds with anti-cancer activity, better safety and polar groups for further application, five sorafenib analogs with new pyridine-2-amide side chains were designed and synthesized. Preliminary pharmacologic studies showed that these compounds displayed much lower toxicities than that of sorafenib. Among them, compound 10b bearing mercaptoethyl group kept relevant antiproliferation potency compared to sorafenib in Huh7 and Hela cell lines with values of IC50 58.79 and 63.67 M, respectively. As a small molecule inhibitor targeting protein tyrosine kinases, thiol in compound 10b would be an active group to react with maleimide in a mild condition for forming nanoparticles Sorafenib-PEG-DGL, which could be developed as a delivery vehicle to improve the concentration of anti-tumor therapeutic agents in the target cancer tissue and reduce side effects in the next study.
Keywords: antiproliferation; molecular modeling; nanoparticles (NPs); sorafenib analogs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

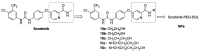
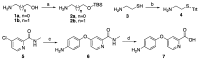

Similar articles
-
Design and synthesis of new potent anticancer benzothiazole amides and ureas featuring pyridylamide moiety and possessing dual B-Raf(V600E) and C-Raf kinase inhibitory activities.Eur J Med Chem. 2016 Jun 10;115:201-16. doi: 10.1016/j.ejmech.2016.02.039. Epub 2016 Feb 18. Eur J Med Chem. 2016. PMID: 27017549
-
Design, synthesis and evaluation of novel 2-(1H-imidazol-2-yl) pyridine Sorafenib derivatives as potential BRAF inhibitors and anti-tumor agents.Eur J Med Chem. 2015 Jan 27;90:170-83. doi: 10.1016/j.ejmech.2014.11.008. Epub 2014 Nov 6. Eur J Med Chem. 2015. PMID: 25461318
-
Design and discovery of thioether and nicotinamide containing sorafenib analogues as multikinase inhibitors targeting B-Raf, B-RafV600E and VEGFR-2.Bioorg Med Chem. 2018 May 15;26(9):2381-2391. doi: 10.1016/j.bmc.2018.03.039. Epub 2018 Apr 3. Bioorg Med Chem. 2018. PMID: 29631788
-
Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma.Eur J Med Chem. 2019 Oct 1;179:916-935. doi: 10.1016/j.ejmech.2019.06.070. Epub 2019 Jun 28. Eur J Med Chem. 2019. PMID: 31306818 Review.
-
The BRAF Status May Predict Response to Sorafenib in Gastrointestinal Stromal Tumors Resistant to Imatinib, Sunitinib, and Regorafenib: Case Series and Review of the Literature.Digestion. 2019;99(2):179-184. doi: 10.1159/000490886. Epub 2018 Sep 4. Digestion. 2019. PMID: 30179868 Review.
Cited by
-
IL-2-loaded liposomes modified with sorafenib derivative exert a synergistic anti-melanoma effect via improving tumor immune microenvironment and enhancing antiangiogenic activity.Asian J Pharm Sci. 2025 Apr;20(2):101020. doi: 10.1016/j.ajps.2025.101020. Epub 2025 Jan 10. Asian J Pharm Sci. 2025. PMID: 40475279 Free PMC article.
References
-
- Whisler R.L., Bagenstose S.E., Newhouse Y.G., Carle K.W. Expression and catalytic activities of protein tyrosine kinases (PTKs) Fyn and Lck in peripheral blood T cells from elderly humans stimulated through the T cell receptor (TCR)/CD3 complex. Mech. Ageing Dev. 1997;98:57–73. doi: 10.1016/S0047-6374(97)00073-0. - DOI - PubMed
-
- Gril B., Palmieri D., Qian Y., Anwar T., Ileva L., Bernardo M., Choyke P., Liewehr D.J., Steinberg S.M., Steeg P.S. The B-Raf status of tumor cells may be a significant determinant of both antitumor and anti-angiogenic effects of pazopanib in xenograft tumor models. Plos ONE. 2011;6:e25625. doi: 10.1371/journal.pone.0025625. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

