Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine
- PMID: 32013041
- PMCID: PMC7075297
- DOI: 10.3390/nano10020226
Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine
Abstract
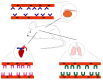
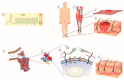
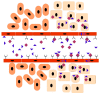
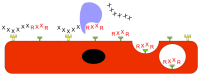
Growth factors, chemokines and cytokines guide tissue regeneration after injuries. However, their applications as recombinant proteins are almost non-existent due to the difficulty of maintaining their bioactivity in the protease-rich milieu of injured tissues in humans. Safety concerns have ruled out their systemic administration. The vascular system provides a natural platform for circumvent the limitations of the local delivery of protein-based therapeutics. Tissue selectivity in drug accumulation can be obtained as organ-specific molecular signatures exist in the blood vessels in each tissue, essentially forming a postal code system ("vascular zip codes") within the vasculature. These target-specific "vascular zip codes" can be exploited in regenerative medicine as the angiogenic blood vessels in the regenerating tissues have a unique molecular signature. The identification of vascular homing peptides capable of finding these unique "vascular zip codes" after their systemic administration provides an appealing opportunity for the target-specific delivery of therapeutics to tissue injuries. Therapeutic proteins can be "packaged" together with homing peptides by expressing them as multi-functional recombinant proteins. These multi-functional recombinant proteins provide an example how molecular engineering gives to a compound an ability to home to regenerating tissue and enhance its therapeutic potential. Regenerative medicine has been dominated by the locally applied therapeutic approaches despite these therapies are not moving to clinical medicine with success. There might be a time to change the paradigm towards systemically administered, target organ-specific therapeutic molecules in future drug discovery and development for regenerative medicine.
Keywords: angiogenesis; cell penetrating peptide; decorin; fibrosis; targeted delivery; transforming growth factor-β (TGF-β), bystander effect, CendR peptide, tissue regeneration, regenerative medicine, hypoxia, neuropilin-1, stem cell; vascular heterogeneity; vascular homing peptide.
Conflict of interest statement
The authors have nothing to declare.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

