Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009
- PMID: 32013142
- PMCID: PMC7037671
- DOI: 10.3390/molecules25030576
Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009
Abstract
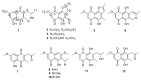
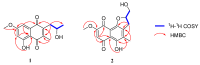
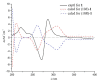
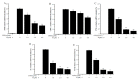
Twelve 1, 4-naphthoquinone derivatives, including two new (1 and 2) and 10 known (3-12), were obtained from endophytic fungus Talaromyces sp. SK-S009 isolated from the fruit of Kandelia obovata. All structures were identified through extensive analysis of the nuclear magnetic resonance (NMR), mass spectrometry (MS) and circular dichroism (CD), as well as by comparison with literature data. These compounds significantly inhibited the lipopolysaccharide (LPS)-induced nitric oxide (NO) production in the murine macrophage cell line (RAW 264.7 cells). The half maximal inhibitory concentration (IC50) values, except for compound 2, were lower than that of indomethacin (26.3 μM). Compound 9 inhibited the LPS-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) mRNA expressions in RAW 264.7 macrophages. Additionally, compound 9 reduced the mRNA levels of pro-inflammatory factors interleukin (IL)1β, IL-6, and tumor necrosis factor (TNF)-α. The results of this study demonstrated that these 1, 4-naphthoquinone derivatives can inhibit LPS-induced inflammation.
Keywords: 1,4-naphthoquinones; Talaromyces sp.; anti-inflammatory; marine fungus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mayer A.M.S., Abimael D.R., Taglialatelascafati O. Marine pharmacology in 2012–2013: Marine compounds with Antibacterial, antidiabetic, antifungal, anti-Inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs. 2017;15:2510–2573. - PMC - PubMed
-
- Cui H., Liu Y., Li J., Huang X., Yan T., Cao W., Liu H., Long Y., She Z. Diaporindenes A−D: Four unusual 2, 3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018;83:11804–11813. doi: 10.1021/acs.joc.8b01738. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

