Tspan8-Tumor Extracellular Vesicle-Induced Endothelial Cell and Fibroblast Remodeling Relies on the Target Cell-Selective Response
- PMID: 32013145
- PMCID: PMC7072212
- DOI: 10.3390/cells9020319
Tspan8-Tumor Extracellular Vesicle-Induced Endothelial Cell and Fibroblast Remodeling Relies on the Target Cell-Selective Response
Abstract
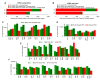
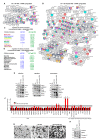
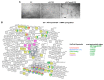
Tumor cell-derived extracellular vesicles (TEX) expressing tetraspanin Tspan8-alpha4/beta1 support angiogenesis. Tspan8-alpha6/beta4 facilitates lung premetastatic niche establishment. TEX-promoted target reprogramming is still being disputed, we explored rat endothelial cell (EC) and lung fibroblast (Fb) mRNA and miRNA profile changes after coculture with TEX. TEX were derived from non-metastatic BSp73AS (AS) or metastatic BSp73ASML (ASML) rat tumor lines transfected with Tspan8 (AS-Tspan8) or Tspan8-shRNA (ASML-Tspan8kd). mRNA was analyzed by deep sequencing and miRNA by array analysis of EC and Fb before and after coculture with TEX. EC and Fb responded more vigorously to AS-Tspan8- than AS-TEX. Though EC and Fb responses differed, both cell lines predominantly responded to membrane receptor activation with upregulation and activation of signaling molecules and transcription factors. Minor TEX-initiated changes in the miRNA profile relied, at least partly, on long noncoding RNA (lncRNA) that also affected chromosome organization and mRNA processing. These analyses uncovered three important points. TEX activate target cell autonomous programs. Responses are initiated by TEX targeting units and are target cell-specific. The strong TEX-promoted lncRNA impact reflects lncRNA shuttling and location-dependent distinct activities. These informations urge for an in depth exploration on the mode of TEX-initiated target cell-specific remodeling including, as a major factor, lncRNA.
Keywords: endothelial cells; fibroblasts; mRNA; message transfer; ncRNA; non-transformed target remodeling; tetraspanin 8; tumor exosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
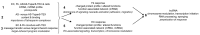







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

