West Nile or Usutu Virus? A Three-Year Follow-Up of Humoral and Cellular Response in a Group of Asymptomatic Blood Donors
- PMID: 32013152
- PMCID: PMC7077259
- DOI: 10.3390/v12020157
West Nile or Usutu Virus? A Three-Year Follow-Up of Humoral and Cellular Response in a Group of Asymptomatic Blood Donors
Abstract
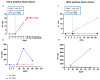
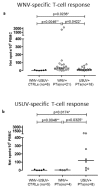
West Nile virus (WNV) and Usutu virus (USUV) are two related arboviruses (genus Flavivirus, family Flaviviridae), with birds as a reservoir and mosquitoes as transmitting vectors. In recent years, WNV epidemiology changed in many European countries with increased frequency of outbreaks posing the issue of virus transmission risks by blood transfusion. USUV emerged for the first time in birds of the Tuscany region (Italy) in 1996 and in 2001 in Austria. While WNV is responsible for both mild and neuroinvasive diseases, USUV infection is usually asymptomatic and neuroinvasive symptoms are rare. Since WNV and USUV co-circulate, the surveillance of WNV allows also the detection of USUV. Due to the great similarity in amino-acid sequence of major surface proteins of the two viruses, a high cross-reactivity can lead to misinterpretation of serological results. Here, we report the results obtained from 54 asymptomatic blood donors during a three-year follow-up showing an unexpected high positivity (46.3%) for USUV. The major obstacle encountered in the differential diagnosis between these two viruses was the high cross-reactivity found in neutralizing antibodies (NT Abs) and, in some cases, a long follow-up was mandatory for a correct diagnosis. Moreover, two new ELISpot assays were developed for a more rapid and specific differential diagnosis, especially in those cases in which NT Abs were not determinant. Using a combination of Enzyme-linked immunospot (ELISpot), molecular, and serological tests, we could identify 25 true positive WNV and 25 true positive USUV blood donors. Our data highlight the importance of raising awareness for increasing USUV infections in endemic countries involved in blood transfusion and organ donation.
Keywords: Lombardia region; Usutu virus; West Nile virus; blood donors; flavivirus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Healy J.M., Reisen W.K., Kramer V.L., Fischer M., Lindsey N.P., Nasci R.S., Macedo P.A., White G., Takahashi R., Khang L., et al. Comparison of the efficiency and cost of West Nile virus surveillance methods in California. Vector Borne Zoonotic Dis. 2015;15:147–155. doi: 10.1089/vbz.2014.1689. - DOI - PMC - PubMed
-
- Rizzo C., Salcuni P., Nicoletti L., Ciufolini M.G., Russo F., Masala R., Frongia O., Finarelli A.C., Gramegna M., Gallo L., et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012;17:20172. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

