Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action
- PMID: 32013158
- PMCID: PMC7070694
- DOI: 10.3390/antiox9020117
Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action
Abstract
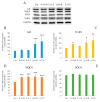
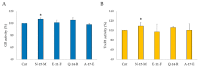
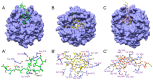
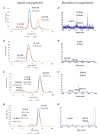
Due to their beneficial properties, fermented foods are considered important constituents of the human diet. They also contain bioactive peptides, health-promoting compounds studied for a wide range of effects. In this work, several antioxidant peptides extracted from fermented milk proteins were investigated. First, enriched peptide fractions were purified and analysed for their antioxidant capacity in vitro and in a cellular model. Subsequently, from the most active fractions, 23 peptides were identified by mass spectrometry MS/MS), synthesized and tested. Peptides N-15-M, E-11-F, Q-14-R and A-17-E were selected for their antioxidant effects on Caco-2 cells both in the protection against oxidative stress and inhibition of ROS production. To define their action mechanism, the activation of the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2(Keap1/Nrf2) pathway was studied evaluating the translocation of Nrf2 from cytosol to nucleus. In cells treated with N-15-M, Q-14-R and A-17-E, a higher amount of Nrf2 was found in the nucleus with respect to the control. In addition, the three active peptides, through the activation of Keap1/Nrf2 pathway, led to overexpression and increased activity of antioxidant enzymes. Molecular docking analysis confirmed the potential ability of N-15-M, Q-14-R and A-17-E to bind Keap1, showing their destabilizing effect on Keap1/Nrf2 interaction.
Keywords: Keap1/Nrf2 pathway; bioactive peptides; natural antioxidants; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Fermented Soy-Derived Bioactive Peptides Selected by a Molecular Docking Approach Show Antioxidant Properties Involving the Keap1/Nrf2 Pathway.Antioxidants (Basel). 2020 Dec 19;9(12):1306. doi: 10.3390/antiox9121306. Antioxidants (Basel). 2020. PMID: 33352784 Free PMC article.
-
Identification and molecular mechanisms of novel antioxidant peptides from two sources of eggshell membrane hydrolysates showing cytoprotection against oxidative stress: A combined in silico and in vitro study.Food Res Int. 2022 Jul;157:111266. doi: 10.1016/j.foodres.2022.111266. Epub 2022 Apr 20. Food Res Int. 2022. PMID: 35761579
-
S-1-propenylmercaptocysteine protects murine hepatocytes against oxidative stress via persulfidation of Keap1 and activation of Nrf2.Free Radic Biol Med. 2019 Nov 1;143:164-175. doi: 10.1016/j.freeradbiomed.2019.07.022. Epub 2019 Jul 23. Free Radic Biol Med. 2019. PMID: 31349040
-
Nuclear factor (erythroid-derived 2)-like 2 (NRF2) drug discovery: Biochemical toolbox to develop NRF2 activators by reversible binding of Kelch-like ECH-associated protein 1 (KEAP1).Arch Biochem Biophys. 2017 Oct 1;631:31-41. doi: 10.1016/j.abb.2017.08.003. Epub 2017 Aug 8. Arch Biochem Biophys. 2017. PMID: 28801166 Review.
-
Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents.Acta Pharm Sin B. 2015 Jul;5(4):285-99. doi: 10.1016/j.apsb.2015.05.008. Epub 2015 Jul 2. Acta Pharm Sin B. 2015. PMID: 26579458 Free PMC article. Review.
Cited by
-
Effect of set-type yoghurt supplemented with the novel probiotic Lantiplantibacillus plantarum 200655 on physicochemical properties and the modulation of oxidative stress-induced damage.Food Sci Biotechnol. 2022 Nov 9;32(3):353-360. doi: 10.1007/s10068-022-01201-0. eCollection 2023 Mar. Food Sci Biotechnol. 2022. PMID: 36778087 Free PMC article.
-
Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation.Molecules. 2023 Dec 6;28(24):7975. doi: 10.3390/molecules28247975. Molecules. 2023. PMID: 38138465 Free PMC article.
-
Value-Added Utilization of Citrus Peels in Improving Functional Properties and Probiotic Viability of Acidophilus-bifidus-thermophilus (ABT)-Type Synbiotic Yoghurt during Cold Storage.Foods. 2022 Sep 2;11(17):2677. doi: 10.3390/foods11172677. Foods. 2022. PMID: 36076870 Free PMC article.
-
Current Trends of Bioactive Peptides-New Sources and Therapeutic Effect.Foods. 2020 Jun 29;9(7):846. doi: 10.3390/foods9070846. Foods. 2020. PMID: 32610520 Free PMC article. Review.
-
Antioxidant and Renin Inhibitory Activities of Peptides from Food Proteins on Hypertension: A Review.Plant Foods Hum Nutr. 2023 Sep;78(3):493-505. doi: 10.1007/s11130-023-01085-3. Epub 2023 Aug 14. Plant Foods Hum Nutr. 2023. PMID: 37578677 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

