Gold Nanoclusters as Electrocatalysts for Energy Conversion
- PMID: 32013164
- PMCID: PMC7075145
- DOI: 10.3390/nano10020238
Gold Nanoclusters as Electrocatalysts for Energy Conversion
Abstract
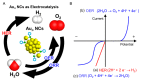
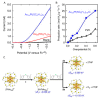
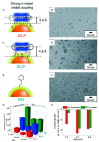
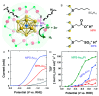
Gold nanoclusters (Aun NCs) exhibit a size-specific electronic structure unlike bulk gold and can therefore be used as catalysts in various reactions. Ligand-protected Aun NCs can be synthesized with atomic precision, and the geometric structures of many Aun NCs have been determined by single-crystal X-ray diffraction analysis. In addition, Aun NCs can be doped with various types of elements. Clarification of the effects of changes to the chemical composition, geometric structure, and associated electronic state on catalytic activity would enable a deep understanding of the active sites and mechanisms in catalytic reactions as well as key factors for high activation. Furthermore, it may be possible to synthesize Aun NCs with properties that surpass those of conventional catalysts using the obtained design guidelines. With these expectations, catalyst research using Aun NCs as a model catalyst has been actively conducted in recent years. This review focuses on the application of Aun NCs as an electrocatalyst and outlines recent research progress.
Keywords: alloy; catalyst; cluster; fuel cells; gold; hydrogen evolution reaction; ligand-protected; oxygen evolution reaction; oxygen reduction reaction; water splitting.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Atomically precise gold nanoclusters as new model catalysts.Acc Chem Res. 2013 Aug 20;46(8):1749-58. doi: 10.1021/ar300213z. Epub 2013 Mar 27. Acc Chem Res. 2013. PMID: 23534692
-
Gold nanoclusters as electrocatalysts: size, ligands, heteroatom doping, and charge dependences.Nanoscale. 2020 May 14;12(18):9969-9979. doi: 10.1039/d0nr00702a. Nanoscale. 2020. PMID: 32167113
-
Atomically Precise Alloy Nanoclusters.Chemistry. 2020 Dec 9;26(69):16150-16193. doi: 10.1002/chem.202001877. Epub 2020 Oct 26. Chemistry. 2020. PMID: 32453462 Review.
-
Doping-mediated excited state dynamics of diphosphine-protected M@Au12 (M = Au, Ir) superatom nanoclusters.Nanoscale. 2024 Jul 25;16(29):14081-14088. doi: 10.1039/d4nr02051k. Nanoscale. 2024. PMID: 39004999
-
Thiolate-Protected Metal Nanoclusters: Recent Development in Synthesis, Understanding of Reaction, and Application in Energy and Environmental Field.Small. 2021 Jul;17(27):e2005328. doi: 10.1002/smll.202005328. Epub 2021 Feb 1. Small. 2021. PMID: 33522090 Review.
Cited by
-
Structural water molecules dominated p band intermediate states as a unified model for the origin on the photoluminescence emission of noble metal nanoclusters: from monolayer protected clusters to cage confined nanoclusters.Sci Technol Adv Mater. 2023 May 15;24(1):2210723. doi: 10.1080/14686996.2023.2210723. eCollection 2023. Sci Technol Adv Mater. 2023. PMID: 37205011 Free PMC article. Review.
-
One-, Two-, and Three-Dimensional Self-Assembly of Atomically Precise Metal Nanoclusters.Nanomaterials (Basel). 2020 Jun 3;10(6):1105. doi: 10.3390/nano10061105. Nanomaterials (Basel). 2020. PMID: 32503177 Free PMC article. Review.
-
Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics.Molecules. 2023 Jul 20;28(14):5531. doi: 10.3390/molecules28145531. Molecules. 2023. PMID: 37513403 Free PMC article. Review.
-
Supramolecular Gold Chemistry: From Atomically Precise Thiolate-Protected Gold Nanoclusters to Gold-Thiolate Nanostructures.Nanomaterials (Basel). 2020 Feb 21;10(2):377. doi: 10.3390/nano10020377. Nanomaterials (Basel). 2020. PMID: 32098101 Free PMC article.
-
Ligand exchange reactions on thiolate-protected gold nanoclusters.Nanoscale Adv. 2021 Apr 6;3(10):2710-2727. doi: 10.1039/d1na00178g. Nanoscale Adv. 2021. PMID: 34046556 Free PMC article. Review.
References
-
- Brust M., Walker M., Bethell D., Schiffrin D.J., Whyman R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994:801–802. doi: 10.1039/C39940000801. - DOI
-
- Kurashige W., Niihori Y., Sharma S., Negishi Y. Precise Synthesis, Functionalization and Application of Thiolate-Protected Gold Clusters. Coord. Chem. Rev. 2016;320:238–250. doi: 10.1016/j.ccr.2016.02.013. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

