Excited State Lifetimes of Sulfur-Substituted DNA and RNA Monomers Probed Using the Femtosecond Fluorescence Up-Conversion Technique
- PMID: 32013184
- PMCID: PMC7037914
- DOI: 10.3390/molecules25030584
Excited State Lifetimes of Sulfur-Substituted DNA and RNA Monomers Probed Using the Femtosecond Fluorescence Up-Conversion Technique
Abstract
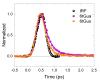
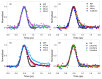
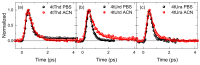
Sulfur-substituted DNA and RNA nucleobase derivatives (a.k.a., thiobases) are an important family of biomolecules. They are used as prodrugs and as chemotherapeutic agents in medical settings, and as photocrosslinker molecules in structural-biology applications. Remarkably, excitation of thiobases with ultraviolet to near-visible light results in the population of long-lived and reactive triplet states on a time scale of hundreds of femtoseconds and with near-unity yields. This efficient nonradiative decay pathway explains the vanishingly small fluorescence yields reported for the thiobases and the scarcity of fluorescence lifetimes in the literature. In this study, we report fluorescence lifetimes for twelve thiobase derivatives, both in aqueous solution at physiological pH and in acetonitrile. Excitation is performed at 267 and 362 nm, while fluorescence emission is detected at 380, 425, 450, 525, or 532 nm. All the investigated thiobases reveal fluorescence lifetimes that decay in a few hundreds of femtoseconds and with magnitudes that depend and are sensitive to the position and degree of sulfur-atom substitution and on the solvent environment. Interestingly, however, three thiopyrimidine derivatives (i.e., 2-thiocytidine, 2-thiouridine, and 4-thiothymidine) also exhibit a small amplitude fluorescence component of a few picoseconds in aqueous solution. Furthermore, the N-glycosylation of thiobases to form DNA or RNA nucleoside analogues is demonstrated as affecting their fluorescence lifetimes. In aqueous solution, the fluorescence decay signals exciting at 267 nm are equal or slower than those collected exciting at 362 nm. In acetonitrile, however, the fluorescence decay signals recorded upon 267 nm excitation are, in all cases, faster than those measured exciting at 362 nm. A comparison to the literature values show that, while both the DNA and RNA nucleobase and thiobase derivatives exhibit sub-picosecond fluorescence lifetimes, the 1ππ* excited-state population in the nucleobase monomers primarily decay back to the ground state, whereas it predominantly populates long-lived and reactive triplet states in thiobase monomers.
Keywords: DNA and RNA derivatives; excited-state dynamics; fluorescence lifetimes; prodrugs; thiobases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The origin of efficient triplet state population in sulfur-substituted nucleobases.Nat Commun. 2016 Oct 5;7:13077. doi: 10.1038/ncomms13077. Nat Commun. 2016. PMID: 27703148 Free PMC article.
-
2,4-Dithiothymine as a potent UVA chemotherapeutic agent.J Am Chem Soc. 2014 Dec 31;136(52):17930-3. doi: 10.1021/ja510611j. Epub 2014 Dec 17. J Am Chem Soc. 2014. PMID: 25506742
-
Base-stacking disorder and excited-state dynamics in single-stranded adenine homo-oligonucleotides.J Phys Chem B. 2012 Aug 30;116(34):10266-74. doi: 10.1021/jp305350t. Epub 2012 Aug 22. J Phys Chem B. 2012. PMID: 22853704
-
Fundamentals of the Intrinsic DNA Fluorescence.Acc Chem Res. 2021 Mar 2;54(5):1226-1235. doi: 10.1021/acs.accounts.0c00603. Epub 2021 Feb 15. Acc Chem Res. 2021. PMID: 33587613 Review.
-
Photochemical and Photodynamical Properties of Sulfur-Substituted Nucleic Acid Bases.Photochem Photobiol. 2019 Jan;95(1):33-58. doi: 10.1111/php.12975. Epub 2018 Sep 9. Photochem Photobiol. 2019. PMID: 29978490 Review.
Cited by
-
Exploring the Influence of Intermolecular Interactions in Prebiotic Chemistry Using Laser Spectroscopy and Calculations.Chemistry. 2022 Jan 3;28(1):e202103636. doi: 10.1002/chem.202103636. Epub 2021 Dec 2. Chemistry. 2022. PMID: 34854511 Free PMC article.
-
Photochemistry of 2-thiooxazole: a plausible prebiotic precursor to RNA nucleotides.Phys Chem Chem Phys. 2022 Sep 14;24(35):21406-21416. doi: 10.1039/d2cp03167a. Phys Chem Chem Phys. 2022. PMID: 36047336 Free PMC article.
-
Ribose Alters the Photochemical Properties of the Nucleobase in Thionated Nucleosides.J Phys Chem Lett. 2021 Jul 22;12(28):6707-6713. doi: 10.1021/acs.jpclett.1c01384. Epub 2021 Jul 14. J Phys Chem Lett. 2021. PMID: 34260253 Free PMC article.
-
Electronic relaxation pathways in thio-acridone and thio-coumarin: two heavy-atom-free photosensitizers absorbing visible light.Phys Chem Chem Phys. 2024 Nov 27;26(46):28980-28991. doi: 10.1039/d4cp03720k. Phys Chem Chem Phys. 2024. PMID: 39552238 Free PMC article.
References
-
- Zheng Q., Xu Y.-Z., Swann P.F. Photochemical cross-linking of λ-Cro repressor to operator DNA containing 4-thiothymine or 6-thioguanine. Nucleos. Nucleot. 1997;16:1799–1803. doi: 10.1080/07328319708006282. - DOI

