Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver
- PMID: 32013203
- PMCID: PMC7076551
- DOI: 10.3390/pharmaceutics12020107
Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver
Abstract
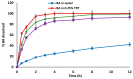
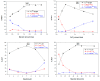
In this study, water-soluble chitosan lactate (CL) was reacted with lactobionic acid (LA), a disaccharide with remarkable affinity to hepatic asialoglycoprotein (ASGP) receptors, to form dual liver-targeting LA-modified-CL polymer for site-specific drug delivery to the liver. The synthesized polymer was used to encapsulate baicalin (BA), a promising bioactive flavonoid with pH-dependent solubility, into ultrahigh drug-loaded nanoparticles (NPs) via the ionic gelation method. The successful chemical conjugation of LA with CL was tested and the formulated drug-loaded LA-modified-CL-NPs were assessed in terms of particle size (PS), encapsulation efficiency (EE) and zeta potential (ZP) using full factorial design. The in vivo biodistribution and pharmacokinetics of the designed NPs were assessed using 99mTc-radiolabeled BA following oral administration to mice and results were compared to 99mTc-BA-loaded-LA-free-NPs and 99mTc-BA solution as controls. Results showed that the chemical modification of CL with LA was successfully achieved and the method of preparation of the optimized NPs was very efficient in encapsulating BA into nearly spherical particles with an extremely high EE exceeding 90%. The optimized BA-loaded-LA-modified-CL-NPs showed an average PS of 490 nm, EE of 93.7% and ZP of 48.1 mV. Oral administration of 99mTc-BA-loaded-LA-modified-CL-NPs showed a remarkable increase in BA delivery to the liver over 99mTc-BA-loaded-LA-free-CL-NPs and 99mTc-BA oral solution. The mean area under the curve (AUC0-24) estimates from liver data were determined to be 11-fold and 26-fold higher from 99mTc-BA-loaded-LA-modified-CL-NPs relative to 99mTc-BA-loaded-LA-free-CL-NPs and 99mTc-BA solution respectively. In conclusion, the outcome of this study highlights the great potential of using LA-modified-CL-NPs for the ultrahigh encapsulation of therapeutic molecules with pH-dependent/poor water-solubility and for targeting the liver.
Keywords: baicalin; chitosan lactate; in vivo biodistribution study; ionic gelation method; lactobionic acid; liver targeting; nanoparticles; radiolabeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Brain targeting of zolmitriptan via transdermal terpesomes: statistical optimization and in vivo biodistribution study by 99mTc radiolabeling technique.Drug Deliv Transl Res. 2023 Dec;13(12):3059-3076. doi: 10.1007/s13346-023-01373-0. Epub 2023 Jun 5. Drug Deliv Transl Res. 2023. PMID: 37273147 Free PMC article.
-
Effect of Size on the Biodistribution and Blood Clearance of Etoposide-Loaded PLGA Nanoparticles.PDA J Pharm Sci Technol. 2011 Mar-Apr;65(2):131-9. PDA J Pharm Sci Technol. 2011. PMID: 21502074
-
A coupled bimodal SPECT-CT imaging and brain kinetics studies of zolmitriptan-encapsulated nanostructured polymeric carriers.Drug Deliv Transl Res. 2018 Jun;8(3):797-805. doi: 10.1007/s13346-017-0474-4. Drug Deliv Transl Res. 2018. PMID: 29380155
-
99mTc-Galactosyl-methylated chitosan.2009 Sep 15 [updated 2009 Dec 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Sep 15 [updated 2009 Dec 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641848 Free Books & Documents. Review.
-
The protective effects of baicalin for respiratory diseases: an update and future perspectives.Front Pharmacol. 2023 Mar 16;14:1129817. doi: 10.3389/fphar.2023.1129817. eCollection 2023. Front Pharmacol. 2023. PMID: 37007037 Free PMC article. Review.
Cited by
-
Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix.Pharmaceutics. 2020 Jul 16;12(7):669. doi: 10.3390/pharmaceutics12070669. Pharmaceutics. 2020. PMID: 32708823 Free PMC article. Review.
-
Intranasal Radioiodinated Ferulic Acid Polymeric Micelles as the First Nuclear Medicine Imaging Probe for ETRA Brain Receptor.Curr Radiopharm. 2024;17(2):209-217. doi: 10.2174/0118744710269885231113070356. Curr Radiopharm. 2024. PMID: 38213167
-
Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer.Pharmaceutics. 2020 Aug 30;12(9):828. doi: 10.3390/pharmaceutics12090828. Pharmaceutics. 2020. PMID: 32872639 Free PMC article.
-
Targeted Fluorescent Polysaccharide Platform for Solute Carrier Protein PCNA Detection and Cervical Squamous Cell Carcinoma Inhibition.J Fluoresc. 2025 Jun 13. doi: 10.1007/s10895-025-04392-x. Online ahead of print. J Fluoresc. 2025. PMID: 40512372
-
Radiolabelling and bioequivalence of modified Tamoxifen solid lipid nanoparticles as a targeted chemotherapeutic drug.Drug Deliv Transl Res. 2025 May 7. doi: 10.1007/s13346-025-01865-1. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40335856
References
-
- Luo J., Dong B., Wang K., Cai S., Liu T., Cheng X., Lei D., Chen Y., Li Y., Kong J., et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE. 2017;12:e0176883. doi: 10.1371/journal.pone.0176883. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

