A Versatile Approach to Access Trimetallic Complexes Based on Trisphosphinite Ligands
- PMID: 32013217
- PMCID: PMC7037439
- DOI: 10.3390/molecules25030593
A Versatile Approach to Access Trimetallic Complexes Based on Trisphosphinite Ligands
Abstract
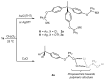
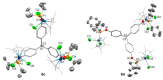
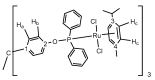
A straightforward method for the preparation of trisphosphinite ligands in one step, using only commercially available reagents (1,1,1-tris(4-hydroxyphenyl)ethane and chlorophosphines) is described. We have made use of this approach to prepare a small family of four trisphosphinite ligands of formula [CH3C{(C6H4OR2)3], where R stands for Ph (1a), Xyl (1b, Xyl = 2,6-Me2-C6H3), iPr (1c), and Cy (1d). These polyfunctional phosphinites allowed us to investigate their coordination chemistry towards a range of late transition metal precursors. As such, we report here the isolation and full characterization of a number of Au(I), Ag(I), Cu(I), Ir(III), Rh(III) and Ru(II) homotrimetallic complexes, including the structural characterization by X-ray diffraction studies of six of these compounds. We have observed that the flexibility of these trisphosphinites enables a variety of conformations for the different trimetallic species.
Keywords: coordination polymer; multidentate ligands; phosphinite; polymetallic; supramolecular chemistry; trimetallic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Luminescent di- and polynuclear organometallic gold(I)-metal (Au2, {Au2Ag}n and {Au2Cu}n) compounds containing bidentate phosphanes as active antimicrobial agents.Chemistry. 2012 Mar 19;18(12):3659-74. doi: 10.1002/chem.201103145. Epub 2012 Feb 14. Chemistry. 2012. PMID: 22334444 Free PMC article.
-
Synthesis and reactivity of fluoro complexes: Part 2. Rhodium(I) fluoro complexes with alkene and phosphine ligands. Synthesis of the first isolated rhodium(I) bifluoride complexes. Structure of [Rh3(mu3-OH)2(COD)(3)](HF2) by X-ray powder diffraction.Inorg Chem. 2004 Sep 6;43(18):5665-75. doi: 10.1021/ic049585e. Inorg Chem. 2004. PMID: 15332818
-
Cyclometalated iridium complexes of bis(aryl) phosphine ligands: catalytic C-H/C-D exchanges and C-C coupling reactions.Inorg Chem. 2013 Jun 3;52(11):6694-704. doi: 10.1021/ic400759r. Epub 2013 May 15. Inorg Chem. 2013. PMID: 23675910
-
Arene Ruthenium(II) Complexes with Phosphorous Ligands as Possible Anticancer Agents.Chimia (Aarau). 2017 Sep 27;71(9):573-579. doi: 10.2533/chimia.2017.573. Chimia (Aarau). 2017. PMID: 30188287 Review.
-
Nucleophilic phosphinidene complexes: access and applicability.Angew Chem Int Ed Engl. 2010 Mar 15;49(12):2102-13. doi: 10.1002/anie.200905689. Angew Chem Int Ed Engl. 2010. PMID: 20157897 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

