Safety Profile of a Multi-Antigenic DNA Vaccine Against Hepatitis C Virus
- PMID: 32013228
- PMCID: PMC7158683
- DOI: 10.3390/vaccines8010053
Safety Profile of a Multi-Antigenic DNA Vaccine Against Hepatitis C Virus
Abstract
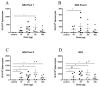
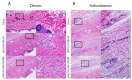
Despite direct acting antivirals (DAAs) curing >95% of individuals infected with hepatitis C (HCV), in order to achieve the World Health Organization HCV Global Elimination Goals by 2030 there are still major challenges that need to be overcome. DAAs alone are unlikely to eliminate HCV in the absence of a vaccine that can limit viral transmission. Consequently, a prophylactic HCV vaccine is necessary to relieve the worldwide burden of HCV disease. DNA vaccines are a promising vaccine platform due to their commercial viability and ability to elicit robust T-cell-mediated immunity (CMI). We have developed a novel cytolytic DNA vaccine that encodes non-structural HCV proteins and a truncated mouse perforin (PRF), which is more immunogenic than the respective canonical DNA vaccine lacking PRF. Initially we assessed the ability of the HCV pNS3-PRF and pNS4/5-PRF DNA vaccines to elicit robust long-term CMI without any adverse side-effects in mice. Interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assay was used to evaluate CMI against NS3, NS4 and NS5B in a dose-dependent manner. This analysis showed a dose-dependent bell-curve of HCV-specific responses in vaccinated animals. We then thoroughly examined the effects associated with reactogenicity of cytolytic DNA vaccination with the multi-antigenic HCV DNA vaccine (pNS3/4/5B). Hematological, biochemical and histological studies were performed in male Sprague Dawley rats with a relative vaccine dose 10-20-fold higher than the proposed dose in Phase I clinical studies. The vaccine was well tolerated, and no toxicity was observed. Thus, the cytolytic multi-antigenic DNA vaccine is safe and elicits broad memory CMI.
Keywords: DNA vaccine; Hepatitis C Virus; Pre-clinical; cell death; immune breadth; pathology; perforin; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A Multiantigenic DNA Vaccine That Induces Broad Hepatitis C Virus-Specific T-Cell Responses in Mice.J Virol. 2015 Aug;89(15):7991-8002. doi: 10.1128/JVI.00803-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018154 Free PMC article.
-
Induction of Genotype Cross-Reactive, Hepatitis C Virus-Specific, Cell-Mediated Immunity in DNA-Vaccinated Mice.J Virol. 2018 Mar 28;92(8):e02133-17. doi: 10.1128/JVI.02133-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29437963 Free PMC article.
-
Single-Dose Vaccination with a Hepatotropic Adeno-associated Virus Efficiently Localizes T Cell Immunity in the Liver with the Potential To Confer Rapid Protection against Hepatitis C Virus.J Virol. 2019 Sep 12;93(19):e00202-19. doi: 10.1128/JVI.00202-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292249 Free PMC article.
-
Hepatitis C Vaccines, Antibodies, and T Cells.Front Immunol. 2018 Jun 28;9:1480. doi: 10.3389/fimmu.2018.01480. eCollection 2018. Front Immunol. 2018. PMID: 30002657 Free PMC article. Review.
-
The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control.J Hepatol. 2016 Oct;65(1 Suppl):S2-S21. doi: 10.1016/j.jhep.2016.07.035. J Hepatol. 2016. PMID: 27641985 Review.
Cited by
-
Hepatitis C virus DNA vaccines: a systematic review.Virol J. 2021 Dec 13;18(1):248. doi: 10.1186/s12985-021-01716-8. Virol J. 2021. PMID: 34903252 Free PMC article.
-
Cardiovascular adverse effects of immunotherapy in cancer: insights and implications.Front Oncol. 2025 Jun 18;15:1601808. doi: 10.3389/fonc.2025.1601808. eCollection 2025. Front Oncol. 2025. PMID: 40606990 Free PMC article. Review.
-
Minicircle-based vaccine induces potent T-cell and antibody responses against hepatitis C virus.Sci Rep. 2024 Nov 4;14(1):26698. doi: 10.1038/s41598-024-78049-3. Sci Rep. 2024. PMID: 39496832 Free PMC article.
-
HCV Core/NS3 Protein Immunization with "N-Terminal Heat Shock gp96 Protein (rNT (gp96))" Induced Strong and Sustained Th1-Type Cytokines in Immunized Mice.Vaccines (Basel). 2021 Mar 3;9(3):215. doi: 10.3390/vaccines9030215. Vaccines (Basel). 2021. PMID: 33802466 Free PMC article.
-
A novel liposome-polymer hybrid nanoparticles delivering a multi-epitope self-replication DNA vaccine and its preliminary immune evaluation in experimental animals.Nanomedicine. 2021 Jul;35:102338. doi: 10.1016/j.nano.2020.102338. Epub 2020 Nov 13. Nanomedicine. 2021. PMID: 33197626 Free PMC article.
References
-
- Marcellin P., Asselah T., Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47–S56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

