Reconstituting the Mammalian Apoptotic Switch in Yeast
- PMID: 32013249
- PMCID: PMC7073680
- DOI: 10.3390/genes11020145
Reconstituting the Mammalian Apoptotic Switch in Yeast
Abstract
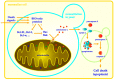
Proteins of the Bcl-2 family regulate the permeabilization of the mitochondrial outer membrane that represents a crucial irreversible step in the process of induction of apoptosis in mammalian cells. The family consists of both proapoptotic proteins that facilitate the membrane permeabilization and antiapoptotic proteins that prevent it in the absence of an apoptotic signal. The molecular mechanisms, by which these proteins interact with each other and with the mitochondrial membranes, however, remain under dispute. Although yeast do not have apparent homologues of these apoptotic regulators, yeast cells expressing mammalian members of the Bcl-2 family have proved to be a valuable model system, in which action of these proteins can be effectively studied. This review focuses on modeling the activity of proapoptotic as well as antiapoptotic proteins of the Bcl-2 family in yeast.
Keywords: BH3-only proteins; Bax; Bcl-2 protein family; Bcl-XL; Saccharomyces cerevisiae; apoptosis; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

