Interplay between BRCA1 and GADD45A and Its Potential for Nucleotide Excision Repair in Breast Cancer Pathogenesis
- PMID: 32013256
- PMCID: PMC7037490
- DOI: 10.3390/ijms21030870
Interplay between BRCA1 and GADD45A and Its Potential for Nucleotide Excision Repair in Breast Cancer Pathogenesis
Abstract
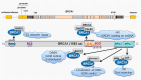
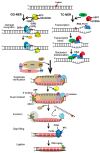
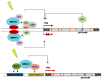
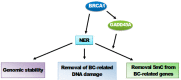
A fraction of breast cancer cases are associated with mutations in the BRCA1 (BRCA1 DNA repair associated, breast cancer type 1 susceptibility protein) gene, whose mutated product may disrupt the repair of DNA double-strand breaks as BRCA1 is directly involved in the homologous recombination repair of such DNA damage. However, BRCA1 can stimulate nucleotide excision repair (NER), the most versatile system of DNA repair processing a broad spectrum of substrates and playing an important role in the maintenance of genome stability. NER removes carcinogenic adducts of diol-epoxy derivatives of benzo[α]pyrene that may play a role in breast cancer pathogenesis as their accumulation is observed in breast cancer patients. NER deficiency was postulated to be intrinsic in stage I of sporadic breast cancer. BRCA1 also interacts with GADD45A (growth arrest and DNA damage-inducible protein GADD45 alpha) that may target NER machinery to actively demethylate genome sites in order to change the expression of genes that may be important in breast cancer. Therefore, the interaction between BRCA1 and GADD45 may play a role in breast cancer pathogenesis through the stimulation of NER, increasing the genomic stability, removing carcinogenic adducts, and the local active demethylation of genes important for cancer transformation.
Keywords: BRCA1; DNA demethylation; GADD45A; NER; breast cancer; nucleotide excision repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair.Nat Genet. 2002 Sep;32(1):180-4. doi: 10.1038/ng953. Epub 2002 Aug 26. Nat Genet. 2002. PMID: 12195423
-
BRCA1 and p53: compensatory roles in DNA repair.J Mol Med (Berl). 2003 Nov;81(11):700-7. doi: 10.1007/s00109-003-0477-0. Epub 2003 Sep 9. J Mol Med (Berl). 2003. PMID: 13679996 Review.
-
Haploinsufficiency for BRCA1 is associated with normal levels of DNA nucleotide excision repair in breast tissue and blood lymphocytes.BMC Med Genet. 2005 Jun 14;6:26. doi: 10.1186/1471-2350-6-26. BMC Med Genet. 2005. PMID: 15955237 Free PMC article.
-
Differing effects of breast cancer 1, early onset (BRCA1) and ataxia-telangiectasia mutated (ATM) mutations on cellular responses to ionizing radiation.Int J Radiat Biol. 2003 Oct;79(10):817-29. doi: 10.1080/09553000310001610952. Int J Radiat Biol. 2003. PMID: 14630541
-
Human syndromes with genomic instability and multiprotein machines that repair DNA double-strand breaks.Histol Histopathol. 2003 Jan;18(1):225-43. doi: 10.14670/HH-18.225. Histol Histopathol. 2003. PMID: 12507302 Review.
Cited by
-
Aristolochic acids-hijacked p53 promotes liver cancer cell growth by inhibiting ferroptosis.Acta Pharmacol Sin. 2025 Jan;46(1):208-221. doi: 10.1038/s41401-024-01354-0. Epub 2024 Aug 1. Acta Pharmacol Sin. 2025. PMID: 39090392
-
Growth arrest and DNA damage-inducible 45: a new player on inflammatory diseases.Front Immunol. 2025 Feb 27;16:1513069. doi: 10.3389/fimmu.2025.1513069. eCollection 2025. Front Immunol. 2025. PMID: 40083548 Free PMC article. Review.
-
Bioinformatic identification and analysis of immune-related chromatin regulatory genes as potential biomarkers in idiopathic pulmonary fibrosis.Ann Transl Med. 2022 Aug;10(16):896. doi: 10.21037/atm-22-3700. Ann Transl Med. 2022. PMID: 36111015 Free PMC article.
-
Liquiritigenin decreases tumorigenesis by inhibiting DNMT activity and increasing BRCA1 transcriptional activity in triple-negative breast cancer.Exp Biol Med (Maywood). 2021 Feb;246(4):459-466. doi: 10.1177/1535370220957255. Epub 2020 Sep 17. Exp Biol Med (Maywood). 2021. PMID: 32938226 Free PMC article.
-
CircDNA2-Educated YTHDF2 Phase Separation Promotes PM2.5-Induced Malignant Transformation Through the Blunting of GADD45A Expression.Adv Sci (Weinh). 2025 Apr;12(14):e2410532. doi: 10.1002/advs.202410532. Epub 2025 Jan 17. Adv Sci (Weinh). 2025. PMID: 39823477 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

