GM1 Ganglioside Is A Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration
- PMID: 32013258
- PMCID: PMC7037093
- DOI: 10.3390/ijms21030868
GM1 Ganglioside Is A Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration
Abstract
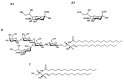
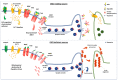
Many species of ganglioside GM1, differing for the sialic acid and ceramide content, have been characterized and their physico-chemical properties have been studied in detail since 1963. Scientists were immediately attracted to the GM1 molecule and have carried on an ever-increasing number of studies to understand its binding properties and its neurotrophic and neuroprotective role. GM1 displays a well balanced amphiphilic behavior that allows to establish strong both hydrophobic and hydrophilic interactions. The peculiar structure of GM1 reduces the fluidity of the plasma membrane which implies a retention and enrichment of the ganglioside in specific membrane domains called lipid rafts. The dynamism of the GM1 oligosaccharide head allows it to assume different conformations and, in this way, to interact through hydrogen or ionic bonds with a wide range of membrane receptors as well as with extracellular ligands. After more than 60 years of studies, it is a milestone that GM1 is one of the main actors in determining the neuronal functions that allows humans to have an intellectual life. The progressive reduction of its biosynthesis along the lifespan is being considered as one of the causes underlying neuronal loss in aged people and severe neuronal decline in neurodegenerative diseases. In this review, we report on the main knowledge on ganglioside GM1, with an emphasis on the recent discoveries about its bioactive component.
Keywords: GM1 ganglioside; GM1 in neurodegeneration; GM1 in neuronal development; GM1 neuronal function; GM1 oligosaccharide function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Svennerholm L. The Gangliosides. J. Lipid Res. 1964;5:145–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

