Newborn screening for Morquio disease and other lysosomal storage diseases: results from the 8-plex assay for 70,000 newborns
- PMID: 32014045
- PMCID: PMC6998831
- DOI: 10.1186/s13023-020-1322-z
Newborn screening for Morquio disease and other lysosomal storage diseases: results from the 8-plex assay for 70,000 newborns
Abstract
Background: The necessity of early treatment for lysosomal storage diseases (LSDs) has triggered the development of newborn screening for LSDs in recent years. Here we report the first 70,000 newborns screened for Mucopolysaccharidosis (MPS) type 4A (Morquio syndrome) and other LSDs by an 8-plex assay including the original 4-plex LSD screening tandem mass spectrometry (MS/MS) assay for Pompe disease, Fabry disease, Gaucher disease, and MPS I disease.
Methods: The additional reaction for MPS II, MPS 3B, MPS 4A, and MPS 6 enzymes was performed separately from the 4-plex reaction. The two reactions were quenched and extracted, then combined before carrying out a single 2-min UPLC-MS/MS analysis.
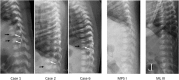
Results: From Mar. 2018 to Apr. 2019, 73,743 newborns were screened with the 8-plex LSD screening assay. The 8-plex assay revealed a better analytical precision than the previous 4-plex assay possibly because the 8-plex was carried out using UPLC-MS/MS. Six newborns were found to have low MPS-4A enzyme (N-acetylgalactosamine-6-sulfatase) activity and biallelic GALNS pathogenic mutations in trans; these patients are presumably affected with MPS4A, making an incidence of one in 12,291 (95% confident interval (CI): 5633-26,817). One mutation, c.857C > T (p.T286 M) of the GALNS gene, accounted 5 of the 12 mutated alleles. These newborns had immature vertebral bodies at 1 month of age, and one case was treated with elosulfase alfa 2 mg/kg/week starting from 4 months of age. Among other MPSs screened, one case of MPS I, 3 cases of MPS II, and 3 cases of MPS 3B were detected. One case of mucolipidosis type III was also diagnosed. In conjunction with another 9 patients of Pompe disease, Gaucher disease, and classical Fabry disease, making an incidence of LSDs as one in 3206 newborns (95% CI: 2137 - 4811). The one with infantile-onset Pompe disease and the one with Gaucher disease were treated since the age of 8 days and 41 days respectively.
Conclusions: Routine newborn screening of MPS 4A and other LSDs were made possible by the 8-plex LSD screening assay. However, detailed phenotype prediction and the time to start treatment will need further elucidation.
Keywords: Lysosomal storage disease; MPS4A; Morquio disease; Mucopolysaccharidosis; Multiplex newborn screening; Tandem mass spectrometry.
Conflict of interest statement
YHC and WLH have received consulting and advisor fees from Biomarin and Sanofi, and receive research funding from Biomarin and Sanofi. M. H. Gelb serves as a consultant for PerkinElmer and gets funding from Takeda, Biomarin, Ultrageneyx, The Legacy of Angels Foundation and CureGM1 Foundation. Others report no competing interests.
Figures
References
-
- Liu Y, Yi F, Kumar AB, Kumar Chennamaneni N, Hong X, Scott CR, et al. Multiplex tandem mass spectrometry enzymatic activity assay for newborn screening of the Mucopolysaccharidoses and type 2 neuronal Ceroid Lipofuscinosis. Clin Chem. 2017;63(6):1118–1126. doi: 10.1373/clinchem.2016.269167. - DOI - PMC - PubMed
-
- Kumar AB, Masi S, Ghomashchi F, Chennamaneni NK, Ito M, Scott CR, et al. Tandem mass spectrometry has a larger analytical range than fluorescence assays of Lysosomal enzymes: application to newborn screening and diagnosis of Mucopolysaccharidoses types II, IVA, and VI. Clin Chem. 2015;61(11):1363–1371. doi: 10.1373/clinchem.2015.242560. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

