Genetic and histologic spatiotemporal evolution of recurrent, multifocal, multicentric and metastatic glioblastoma
- PMID: 32014051
- PMCID: PMC6998196
- DOI: 10.1186/s40478-020-0889-x
Genetic and histologic spatiotemporal evolution of recurrent, multifocal, multicentric and metastatic glioblastoma
Abstract
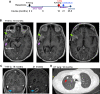
Glioblastoma is the most frequent and aggressive primary brain tumor, characterized by extensive brain invasion and rarely, systemic metastases. The pathogenesis of metastatic glioblastoma is largely unknown. We present the first integrated clinical/histologic/genetic analysis of 5 distinct brain and lung foci from a unique case of recurrent, multifocal, multicentric and metastatic glioblastoma. The initial right frontotemporal gliosarcoma received standard surgical/chemoradiation therapy and recurred 1.5 years later, co-occurring with three additional masses localized to the ipsilateral temporal lobe, cerebellum and lung. Synchronous metastatic lung carcinoma was suspected in this long-term smoker patient with family history of cancer. However, glioblastoma was confirmed in all tumors, although with different morphologic patterns, including ependymomatous and epithelioid. Genomic profiling revealed a germline FANCD2 variant of unknown significance, and a 4-gene somatic mutation signature shared by all tumors, consisting of TERT promoter and PTEN, RB1 and TP53 tumor suppressor mutations. Additional GRIN2A and ATM heterozygous mutations were selected in the cerebellar and lung foci, but were variably present in the supratentorial foci, indicating reduced post-therapeutic genetic evolution in brain foci despite morphologic variability. Significant genetic drift characterized the lung metastasis, likely explaining the known resistance of circulating glioblastoma cells to systemic seeding. MET overexpression was detected in the initial gliosarcoma and lung metastasis, possibly contributing to invasiveness. This comprehensive analysis sheds light on the temporospatial evolution of glioblastoma and underscores the importance of genetic testing for diagnosis and personalized therapy.
Keywords: Epithelioid; Gliosarcoma; Invasion; Metastatic glioblastoma; Multicentric; Multifocal; Next generation sequencing; PTEN; Recurrent.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

