Comparative Analysis of Cellular Immune Responses in Conventional and SPF Olive Baboons (Papio anubis)
- PMID: 32014083
- PMCID: PMC7137550
- DOI: 10.30802/AALAS-CM-19-000035
Comparative Analysis of Cellular Immune Responses in Conventional and SPF Olive Baboons (Papio anubis)
Abstract
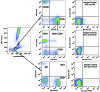
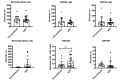
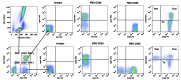
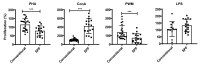
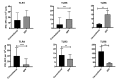
Olive baboons (P. anubis) have provided a useful model of human diseases and conditions, including cardiac, respiratory, and infectious diseases; diabetes; and involving genetics, immunology, aging, and xenotransplantation. The development of a immunologically defined SPF baboons has advanced research further, especially for studies involving the immune system and immunosuppression. In this study, we compare normal immunologic changes of PBMC subsets, and their function in age-matched conventional and SPF baboons. Our results revealed that both groups have comparable numbers of different lymphocyte subsets, but phenotypic differences in central and effector memory T-cell subsets are more pronounced in CD4+ T cells. Despite equal proportions of CD3+ T cells among the conventional and SPF baboons, PBMC from the conventional group showed greater proliferative responses to phytohemagglutinin and pokeweed mitogen and higher numbers of IFNγ-producing cells after stimulation with concanavalin A or pokeweed mitogen, whereas plasma levels of the inflammatory cytokine TNFα were significantly higher in SPF baboons. Exposure of PBMC from conventional baboons to various Toll-like (TLR) ligands, including TLR3, TLR4, and TLR8, yielded increased numbers of IFNγ producing cells, whereas PBMC from SPF baboons stimulated with TLR5 or TLR6 ligand had more IFNγ-producing cells. These findings suggest that although lymphocyte subsets share many phenotypic and functional similarities in conventional and SPF baboons, specific differences in the immune function of lymphocytes could differentially influence the quality and quantity of their innate and adaptive immune responses. These differences should be considered in interpreting experimental outcomes, specifically in studies measuring immunologic endpoints.
Figures


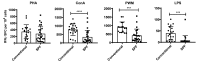

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

