Effect of Chronic Vitamin D Deficiency on the Development and Severity of DSS-Induced Colon Cancer in Smad3-/- Mice
- PMID: 32014085
- PMCID: PMC7137544
- DOI: 10.30802/AALAS-CM-19-000021
Effect of Chronic Vitamin D Deficiency on the Development and Severity of DSS-Induced Colon Cancer in Smad3-/- Mice
Abstract
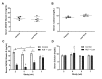
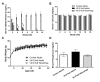
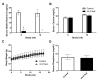
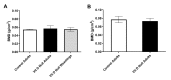
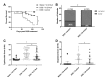
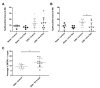
Both human epidemiologic data and animal studies suggest that low serum vitamin D increases the risk of inflammatory bowel disease (IBD) and consequently IBD-associated colorectal cancer. We tested the hypothesis that vitamin D deficiency increases the risk for colitis-associated colon cancer (CAC) by using an established CAC mouse model, 129-Smad3tm1Par/J (Smad3-/-) mice, which have defective transforming growth factor β-signaling and develop colitis and CAC after the administration of dextran sodium sulfate (DSS). After determining a dietary regimen that induced chronic vitamin D deficiency in Smad3-/- mice, we assessed the effects of vitamin D deficiency on CAC. Decreasing dietary vitamin D from 1 IU/g diet (control diet) to 0.2 IU /g diet did not decrease serum 25-hydroxyvitamin D (25(OH)D) levels in Smad3-/- mice. A diet devoid of vitamin D (< 0.02 IU/g diet [no added vitamin D]; vitamin D-null) significantly decreased serum 25(OH)D levels in mice after 2 wk (null compared with control diet: 8.4 mg/mL compared with 12.2 ng/mL) and further decreased serum levels to below the detection limit after 9 wk but did not affect weight gain, serum calcium levels, bone histology, or bone mineral density. In light of these results, Smad3-/- mice were fed a vitamin D-null diet and given DSS to induce colitis. Unexpectedly, DSS-treated Smad3-/- mice fed a vitamin D-null diet had improved survival, decreased colon tumor incidence (8% compared with 36%), and reduced the incidence and severity of colonic dysplasia compared with mice fed the control diet. These effects correlated with increased epithelial cell proliferation and repair early in the disease, perhaps reducing the likelihood of developing chronic colitis and progression to cancer. Our results indicate that vitamin D deficiency is beneficial in some cases of CAC, possibly by promoting intestinal healing.
Figures
References
-
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. 1999. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987. 10.1210/endo.140.11.7110. - DOI - PubMed
-
- Ananthakrishnan AN, Cheng SC, Cai T, Cagan A, Gainer VS, Szolovits P, Shaw SY, Churchill S, Karlson EW, Murphy SN, Kohane I, Liao KP. 2014. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 12:821–827. 10.1016/j.cgh.2013.10.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

