Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle
- PMID: 32014109
- PMCID: PMC7000218
- DOI: 10.7554/eLife.51107
Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle
Abstract
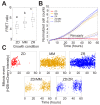
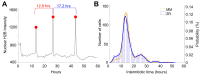
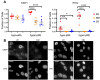
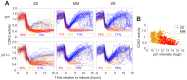
Zinc is widely recognized as essential for growth and proliferation, yet the mechanisms of how zinc deficiency arrests these processes remain enigmatic. Here we induce subtle zinc perturbations and track asynchronously cycling cells throughout division using fluorescent reporters, high throughput microscopy, and quantitative analysis. Zinc deficiency induces quiescence and resupply stimulates synchronized cell-cycle reentry. Monitoring cells before and after zinc deprivation we found the position of cells within the cell cycle determined whether they either went quiescent or entered another cell cycle but stalled in S-phase. Stalled cells exhibited prolonged S-phase, were defective in DNA synthesis and had increased DNA damage levels, suggesting a role for zinc in maintaining genome integrity. Finally, we demonstrate zinc deficiency-induced quiescence occurs independently of DNA-damage response pathways, and is distinct from mitogen removal and spontaneous quiescence. This suggests a novel pathway to quiescence and reveals essential micronutrients play a role in cell cycle regulation.
Keywords: cell biology; cell cycle regulation; human; live cell imaging; micronutrient; quantitative image analysis; quiescence; zinc deficiency.
© 2020, Lo et al.
Conflict of interest statement
ML, LD, JW, SJ, AP No competing interests declared
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

