The Interplay of the Extracellular Matrix and Stromal Cells as a Drug Target in Stroma-Rich Cancers
- PMID: 32014341
- PMCID: PMC8812124
- DOI: 10.1016/j.tips.2020.01.001
The Interplay of the Extracellular Matrix and Stromal Cells as a Drug Target in Stroma-Rich Cancers
Abstract
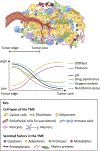
The tumor microenvironment (TME) is a complex neighborhood that consists of immune cells, fibroblasts, pericytes, adipocytes, endothelial and neuronal cells, and the extracellular matrix proteins. TME also consists of physical factors, such as oxygen availability, changing pH, interstitial fluid pressure, and tissue stiffness. As cancer progresses, the physical properties and the cells in the TME change significantly, impacting the efficacy of the therapies and modulating drug resistance. This has led to the development of several new treatments targeting the TME. This review focuses on recent advances on the role of TME in drug resistance, with a particular focus on the ongoing clinical trials aiming at disrupting the TME- and the extracellular matrix-mediated protection against therapies.
Keywords: cancer-associated fibroblasts; clinical trials; drug resistance; extracellular matrix; stroma-cancer crosstalk; tumor microenvironment.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, and Kimmelman AC, Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature, 2016. 536(7617): p. 479–83. - PMC - PubMed
-
- Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, Tumanov S, Allen-Petersen BL, Link J, Kendsersky ND, Vringer E, Schug M, Novo D, Hwang RF, Evans RM, Nixon C, Dorrell C, Morton JP, Norman JC, Sears RC, Kamphorst JJ, and Sherman MH, A Stromal Lysolipid-Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer Discov, 2019. 9(5): p. 617–627. - PMC - PubMed
-
- Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J, Sajjakulnukit P, Hong HS, Kremer DM, Nelson BS, Kemp S, Zhang L, Chang D, Biankin A, Shi J, Frankel TL, Crawford HC, Morton JP, Pasca di Magliano M, and Lyssiotis CA, Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab, 2019. 29(6): p. 1390–1399 e6. - PMC - PubMed
-
- Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, Ando K, Weng L, Ishihara S, Ponik SM, Conklin MW, Haga H, Nagasaka A, Miyata T, Matsuyama M, Kobayashi T, Fujii T, Yamada S, Yamaguchi J, Wang T, Woods SL, Worthley D, Shimamura T, Fujishiro M, Hirooka Y, Takahashi M, and Enomoto A, Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res, 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

