The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19
- PMID: 32014447
- PMCID: PMC7036015
- DOI: 10.1016/j.jcyt.2019.12.004
The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19
Abstract
Thirty years after initial publications of the concept of a chimeric antigen receptor (CAR), the U.S. Food and Drug Administration (FDA) approved the first anti-CD19 CAR T-cell therapy. Unlike other immunotherapies, such as immune checkpoint inhibitors and bispecific antibodies, CAR T cells are unique as they are "living drugs," that is, gene-edited killer cells that can recognize and kill cancer. During these 30 years of development, the CAR construct, T-cell manufacturing process, and clinical patient management have gone through rounds of failures and successes that drove continuous improvement. Tisagenlecleucel was the first gene therapy to receive approval from the FDA for any indication. The initial approval was for relapsed or refractory (r/r) pediatric and young-adult B-cell acute lymphoblastic leukemia in August 2017 and in May 2018 for adult r/r diffuse large B-cell lymphoma. Here we review the preclinical and clinical development of what began as CART19 at the University of Pennsylvania and later developed into tisagenlecleucel.
Keywords: CART; CART19; CTL019, tisagenlecleucel; axicabtagene ciloleucel; chimeric antigen receptor T cells.
Copyright © 2019 International Society for Cell and Gene Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
P.B.: Unrestricted research grant from Novartis Healthcare Denmark contributed to his institution. M.R.: research funding from Novartis; inventor in patents involving the use of CART immunotherapy for cancer. Consultant/advisor Nanostring, Abclon B.L.L.: Scientific Advisory Board of Avectas, ThermoFisher Viral Vector Systems (formerly Brammer Bio), Cure Genetics, Immuneel, Incysus, Ori Biotech, Vycellix, consultancy fees CRC Oncology, licensed intellectual property to Novartis Pharmaceuticals Corporation and Tmunity Therapeutics, and co-Founder, equity holder of Tmunity Therapeutics.
Figures
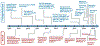

References
-
- Walker RE, Bechtel CM, Natarajan V, Baseler M, Hege KM, Metcalf JA, Stevens R, Hazen A, Blaese RM, Chen CC, Leitman SF, Palensky J, Wittes J, Davey RT Jr., Falloon J, Polis MA, Kovacs JA, Broad DF, Levine BL, Roberts MR, Masur H, Lane HC, Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection, Blood 96(2) (2000) 467–74. - PubMed
-
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM, Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects, Blood 96(3) (2000) 785–93. - PubMed
-
- Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA, Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer, Journal for immunotherapy of cancer 5 (2017) 22. - PMC - PubMed
-
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P, A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer, Clinical cancer research : an official journal of the American Association for Cancer Research 12(20 Pt 1) (2006) 6106–15. - PMC - PubMed
-
- Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E, Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(13) (2006) e20–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

